Pyogenic Infections
with musculoskeletal infections in general. The potential long-term
consequences of these infections can have much more serious
repercussions for affected patients, however, including neurologic
compromise and death. Infection of the spine is usually an indolent
process and often can be well tolerated if diagnosed early. Commonly,
patients have vague back pain that is not evaluated fully until weeks
or months after it began. The profound consequences and the indistinct
clinical presentations of spinal infections highlight the importance of
maintaining a reasonable index of suspicion. Occasionally, precipitous
deterioration occurs, causing patients to present with sepsis,
pathologic fracture, and neurologic compromise. The incidence of spinal
infections is increasing, likely owing to an increase in spinal
procedures, more immunocompromised patients, and escalating numbers of
invasive procedures.
as discitis, pyogenic osteomyelitis, or epidural abscess. Pyogenic
spinal infections usually arise through hematogenous or contiguous
(direct) spread. Hematogenous spread, the most common pathway, involves
seeding of the spinal elements through bacteremia from unrelated
infections. Examples of distant sources include urologic, respiratory,
and skin infections. Direct or contiguous spread may occur through
posttraumatic inoculation, overlying decubitus ulcers, or adjacent
infections (i.e., retropharyngeal or retroperitoneal abscesses), among
others.
variety of potential sources. Iatrogenic causes represent a substantial
portion of the problem. Potential liabilities include indwelling
catheters; invasive spinal procedures, such as surgery or injections;
and pharmacologic immunosuppression. Urologic procedures have a
well-known association with bacteremia and subsequent seeding of the
spinal elements.
bimodal, with a small peak between 10 and 20 years of age and a large
peak in the elderly population (>50 years old). More recently, a new
peak has been developing in the young adult group, possibly
corresponding to increased rates of infection with human
immunodeficiency virus (HIV) and intravenous drug abuse. Males are
affected more commonly than females in all age groups, comprising 60%
to 80% of the infected population. Other important risk factors include
(see also Table 8-1):
-
Diabetes
-
Alcohol abuse
-
Organ transplantation
responsible for most spinal infections in the younger age group. There
is no indication that discitis is more or less prevalent currently. In
developed countries, vertebral osteomyelitis constitutes 2% to 8% of
all cases of osteomyelitis, ranking it a distant third behind femoral
and tibial infections. Epidural
abscesses tend to occur in elderly patients after spinal injections and other invasive procedures.
|
TABLE 8-1 RISK FACTORS FOR PYOGENIC SPINAL INFECTIONS
|
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
infections in general is increasing, likely due to an enlarging elderly
population, more immunocompromised patients, and intravenous drug
abuse. Although there has been a presumed increased incidence in more
recent years, no definitive evidence supporting this conclusion exists
in the literature. The true dynamics are obscured further by a
considerable improvement in detecting infections.
-
Staphylococcus aureus
is the most common and traditionally almost always the cause of spinal
infections, but with changes in risk factor dynamics it now is isolated
in only 50% to 60% of cases. -
Pseudomonas more commonly is associated with intravenous drug abusers than other patient subgroups.
-
Other gram-negative organisms, such as Escherichia coli, Klebsiella, and Proteus, usually occur through genitourinary pathways.
-
Patients with sickle cell disease are more susceptible to Salmonella.
-
Vertebral osteomyelitis in children can develop from Bartonella henselae infection (cat-scratch disease).
-
Postoperative infections associated with instrumentation often involve coagulase-negative Staphylococcus (S. epidermidis) or other normal skin flora.
-
Other reported organisms include Streptococcus and Acinetobacter species. In the presence of hardware, even traditionally benign organisms are thought to be pathologic.
spine. Most remaining infections occur in the thoracic spine, and only
about 7% involve the cervical spine. The thoracolumbar and lumbosacral
junctions each comprise approximately 5% of spinal infections.
|
TABLE 8-2 ORGANISMS: PYOGENIC SPINAL INFECTIONS
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
complexity of the index surgery. Studies of discectomies and
laminectomies consistently have shown infection rates of less than 1%.
Instrumented posterior lumbar fusions generate an approximately 6% rate
of postoperative infection. Cervical spine surgeries show similar
statistics, with infection rates after instrumented posterior
arthrodesis around 4%. Some specific additional risk factors associated
with postoperative infections are revision surgery, instrumentation,
allogeneic blood transfusion/higher blood loss, longer surgery time,
posterior approach to spine, and congenital deformity.
elucidated clearly. Because most infections are thought to be
hematogenous, current theories are based mainly on vascularity. Early
in life, there is a fairly rich blood supply to the nucleus pulposus.
This blood flows from the adjacent end plates and anulus fibrosus into
this central disc region. Through the aging process, vascularity into
the nucleus pulposus diminishes significantly. As an adult, blood flow
is limited mainly to the end plates and minimally at the peripheral
anulus fibrosus. Nutrition is delivered to the adult disc via diffusion.
the development of infection. Seeding of the disc via hematogenous
spread of bacteria can result in discitis, which tends to be more
prevalent in children. As blood flow to the central disc decreases with
age, pyogenic infections tend to originate within the vertebral bodies.
direct means. When the organisms have involved the deeper wound,
instrumentation may be seeded. Formation of a glycocalyx allows
adherence to metal that often can protect the pathogen from otherwise
bactericidal levels of antibiotics. Implants may have a variable
propensity for this bacterial self-preservation. In a study comparing
metal implants and wound infections after bacterial inoculation,
rabbits showed a much higher infection rate in the stainless steel
group (75%) than in the titanium group (35%). This finding has been
correlated with a higher rate of bacterial adherence to stainless steel
than to titanium.
of focus for infection around the spine. Involvement of the disc alone
commonly has been referred to as discitis. Vertebral osteomyelitis
denotes infection limited to the vertebral body, which eventually may
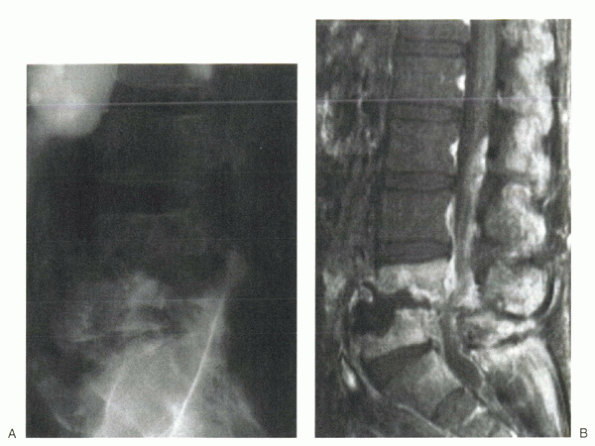
cross the end plate to involve the disc, then adjacent segments (Fig. 8-1). This combination of involvement has been termed spondylodiscitis. Infections localized to the epidural space traditionally have been called epidural abscesses. Frequently, radiographic work-ups occur late in the process and can show diffuse involvement of one or more of these regions.
 |
|
Figure 8-1 Plain radiograph (A) and MRI (B) of spondylodiscitis with adjacent vertebral bodies and the intervening disc involved in the infection.
|
with spinal infection is back pain. The vague nature of this complaint
contributes greatly to the frequent delay in diagnosis. Although the
back pain may occur acutely, it usually has an insidious onset. Of
patients, 50% to 70% have symptoms longer than 3 months before
diagnosis.
with worsening at night, but it also can heighten with activity.
Frequently, pain is accompanied by fever, night sweats, chills, weight
loss, or other constitutional symptoms. Persistent and worsening back
pain in the presence of one or more of these constitutional symptoms
helps differentiate it from typical mechanical low back pain. In
addition, there is often paraspinal muscle spasm with limited back
range of motion due to pain. Involvement of the adjacent psoas muscles
can result in painful hip flexion and extension.
findings have been reported in about 10% of patients. Epidural
abscesses and late, complicated infections tend to be more responsible
for these findings. Neurologic compromise is associated more frequently
with thoracic and cervical spinal infections.
that varies with age. Irritability is common in infants and toddlers,
with occasional difficulty and eventual refusal to walk. Older children
may complain of abdominal or back pain and have spinal rigidity or
localized tenderness on exam. High temperatures are the exception
rather than the rule, with an average temperature of 37.6°C in one
series.
present with wound discharge and fever. These findings usually become
apparent 1 to 2 weeks after surgery. Postoperative infections have
presented nearly 2 years after surgery, however, and may show no more
clinical symptoms than chronic or worsening pain.
work-up of patients suspected of having a spinal infection. White blood
cell (WBC) count generally provides little help because the WBCs are
abnormally elevated in only one third of patients. Epidural abscesses
can cause substantial elevations, however, with an average WBC count of
approximately 22,000 cells/mm3. Epidural abscesses also have been associated with thrombocytopenia.
for systemic inflammatory response and is the most sensitive laboratory
test for spinal infection. The ESR is elevated in 90% to 100% of
children with discitis and approximately 90% of patients with vertebral
osteomyelitis. Normal ESR values range from 0 to 20 mm/h, but some
normal conditions can cause mild elevations. Most series of spinal
infections and associated ESR testing report values greater than 40
mm/h. The ESR normalizes slowly after surgery (≥4 to 6 weeks) and is
less useful when evaluating for postoperative infections.
is the C-reactive protein (CRP) level. CRP is an acute-phase reactant
that accumulates rapidly during infectious or injurious processes. In
contrast to the ESR, the CRP level generally normalizes by 6 to 10 days
after surgery. This characteristic makes it more valuable when
evaluating for infections postoperatively. Additionally, because the
CRP level normalizes relatively quickly, it is used frequently for
longitudinal appraisal of successful treatment. The CRP level is highly
sensitive, and it tends to be more specific than the ESR.
on all patients suspected of spinal infection. Although they are
positive in only one third to three quarters of patients with
documented infection, isolation of the responsible microbes is an
important step in long-term management. Microbial identification yield
also may be improved by culturing other potentially infective sources,
such as sputum or urine.
usually with computed tomography (CT) or fluoroscopic guidance.
Percutaneous biopsy is successful 60% to 96% of the time.
Antimicrobials should be withheld before attempting to obtain biopsy
specimens.
Despite this universal approach, plain x-rays usually are negative
early on in the infectious process and may take 12 weeks to show
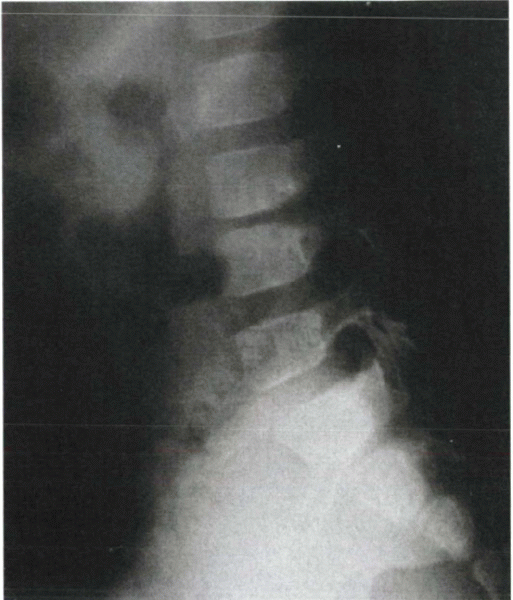
notable changes. An exception occurs in children with discitis. By
several weeks after the onset of symptoms, childhood discitis usually
causes disc space narrowing seen on radiographs (Fig. 8-2). Vertebral disc height can return to normal (more often in younger children), but usually takes several months to a year.
|
TABLE 8-3 RADIOGRAPHIC IMAGING OF PYOGENIC SPINAL INFECTIONS
|
||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||
 |
|
Figure 8-2 Plain radiograph of discitis in a child. Note the classic disc space narrowing at L3-4 and the more subtle loss of lordosis.
|
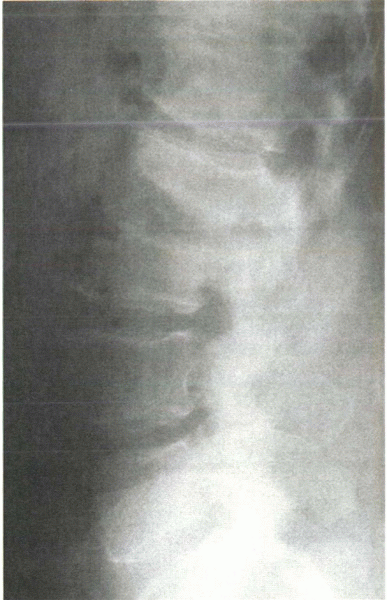
radiographic narrowing of the disc space, but this follows osseous
destruction of the adjacent end plates as shown in Figure 8-3.
This distinguishes the process from most malignancies, which usually
are confined to the vertebral body and do not cross the disc space.
Vertebral body destruction and compression occur later in the process.
Epidural abscesses alone rarely show significant abnormalities on plain
radiographs.
imaging modality of choice. MRI displays abnormalities in the disc,
epidural space, and paraspinal anatomy, and its sensitivity for
detecting infection reportedly is 99%. With infection of the vertebral
body or disc, T1 signal intensity is decreased owing to inflammation
and edema. Such a relative increase in water content causes the T2
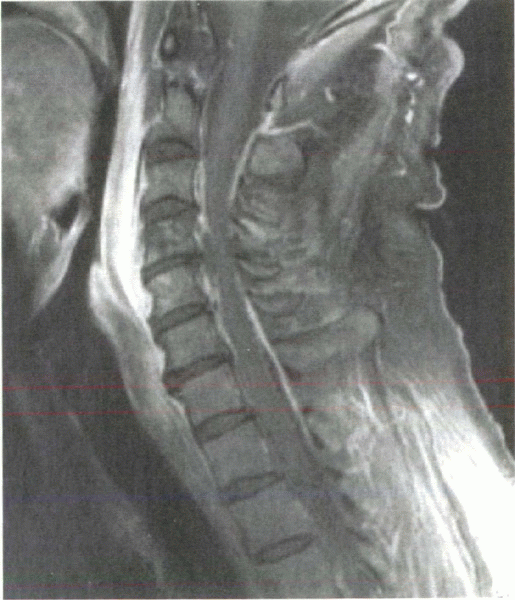
signal intensity to increase. The use of contrast agents (i.e.,
gadolinium) allows even greater accuracy in identifying spinal
infections with MRI (Fig. 8-4). It is useful in detecting epidural abscesses.
spine, with findings of osteolysis more clearly visible than on plain
radiographs. The general quality of bone can be assessed best with this
modality. CT scans often can be extremely valuable when planning
surgical treatment and guiding biopsy procedures around the spine.
if MRI is contraindicated (e.g., pacemaker) or not possible (e.g.,
stainless steel implants, claustrophobia). Nuclear medicine studies
include three-phase technetium-99m, gallium, and indium-labeled WBC
scans. A combined technetium and gallium scan provides the best
sensitivity and specificity. Although indium-labeled WBC scans are
highly specific, a high rate of false-negative results limits its role
in the detection of spinal infection.
 |
|
Figure 8-3 Plain radiograph of infectious end plate rarefaction and narrowing with probable involvement of the intervertebral disc.
|
 |
|
Figure 8-4
T1-weighted MRI with gadolinium contrast enhancement elucidates a cervical epidural abscess within the anterior canal. Note the surrounding contrast halo, which is a classic finding with these infections. |
-
A thorough history and physical examination are done first.
-
Plain radiographs are obtained.
-
Screening laboratory tests always include WBC count, ESR, and CRP.
-
Other initial laboratory studies can be directed by history specifics (e.g., exposure to Mycobacterium tuberculosis).
-
Blood cultures should be obtained initially if the index of suspicion is high.
-
MRI is the next radiographic study of choice, although nuclear medicine studies or CT can be performed when MRI is not feasible.
-
When radiographic and laboratory work-up
has revealed a likely infectious process, a biopsy specimen should be
obtained through percutaneous or open means or directly at the time of
surgery (if indicated).
primary infections, although this treatment should be reserved for
younger and more immunocompetent patients. In one series of 111
patients with vertebral osteomyelitis, the one third who failed
nonoperative treatment tended to be older and immunodeficient. It is
important to obtain the offending microbe via blood cultures or guided
biopsy before beginning antibiotic treatment. Antiobiotic regimens
should be selected based on the organism’s sensitivity profile and
should be begun parenterally with an eventual switch to oral regimens
in some cases.
intravenous antibiotics empirically, even with negative blood cultures
(guided biopsy rarely is indicated). A short (1 to 2 days) period of
bed rest may be beneficial, but further activity restrictions should
not be imposed. While awaiting clinical and laboratory (CRP) responses,
an oral antibiotic regimen is begun. If this trend in improvement
continues on the appropriate oral antibiotics, they are continued for 4
to 6 weeks. Occasionally, use of a brace may be indicated if symptoms
persist. Children almost universally respond to these treatments and
rarely require subsequent surgical intervention.
approached slightly differently. Spinal immobilization plays a larger
role in this population, but early ambulation should be encouraged.
High-dose parenteral antibiotics should be administered for at least 4
to 6 weeks before any switch to oral agents. Infectious disease
specialists can be helpful with optimizing drug regimens, especially
when dealing with virulent or resistant species that may require a
multidrug approach. Elimination of spine pain may occur in nearly 75%
of patients, and spontaneous fusion often occurs.
compromise, nonoperative treatment rarely is indicated. Nonoperative
treatment should be reserved for young, immunocompetent
patients
with minimal radiographic abnormalities and no neurologic changes.
Immobilization and parenteral antibiotics should be used as in
vertebral osteomyelitis, with little hesitancy for surgical
intervention should neurologic changes develop.
abscess or osteomyelitis can be treated nonoperatively. A biopsy
specimen should be obtained, if possible, to isolate the offending
organism and direct the appropriate intravenous antibiotics for
approximately 6 weeks. Orthoses can be considered for pain control, and
close monitoring should be done to detect development of an epidural
abscess or instability (deformity).
-
Failure of nonoperative treatment
-
Need for open biopsy
-
Presence of spinal abscess
-
Sepsis
-
Progressive spinal deformity
-
Refractory spine pain
-
Spinal instability
-
Neurologic compromise
immunodeficiency are relative indications for surgery before a trial of
medical treatment alone. Progressive neurologic deficits usually
necessitate urgent operative intervention. Postoperative spinal
infections after fusion procedures also usually warrant surgical
débridement.
infections are adequate débridement, neural element decompression (if
necessary), and rigid stabilization. Anterior infections, including
vertebral osteomyelitis and discitis, should be approached with an
anterior decompression and débridement. This procedure commonly is
followed by anterior structural bone grafting and a concomitant or
staged posterior instrumentation (Fig. 8-5).
Choices for anterior structural support are numerous, but current
recommendations are for autogenous structural bone graft (e.g., fibula,
rib, tricortical iliac crest). Cortical allograft and vascularized bone
graft also are used, but allografts may harbor residual microbes more
readily. Anterior instrumentation, in addition to bone grafting, has
been successful in the cervical spine when treating infection and may
obviate the need for posterior instrumentation (Fig. 8-6).
Some surgeons now are using metal cages successfully for anterior
reconstruction in the thoracic and lumbar spine without increasing the
risk of recurrent infection. In a preliminary study, titanium cages
were associated with a 58% reduction in hospital time, a decrease in
brace time, an improvement in sagittal alignment, and an 18% reduction
in postoperative complications compared with other methods. Widespread
use of anterior instrumentation has been limited, however, by concerns
over the potential for pathogen incubation on metal implants. Examples
of anterior instrumentation include plates, rods, and metal cage
devices (Fig. 8-7). The addition of posterior
instrumentation allows more rigid spinal fixation, which may lead to
earlier mobilization and diminished pain.
|
TABLE 8-4 SURGICAL INDICATIONS FOR PYOGENIC SPINE INFECTIONS
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||
decompression alone except when directed at posteriorly located
epidural abscesses without anterior spinal involvement. In general,
results have been poor when attempting posterior decompression without
fusion in other circumstances.
anterior/posterior approach, some surgeons advocate posterior
decompression and fusion alone. Decompression of anterior elements and
placement of a strut graft through a posterior approach is more
technically demanding, but results have
been
encouraging in several series. If the posterior spinal elements and
epidural space are free of infection, some surgeons advocate immediate
(rather than delayed) placement of posterior instrumentation.
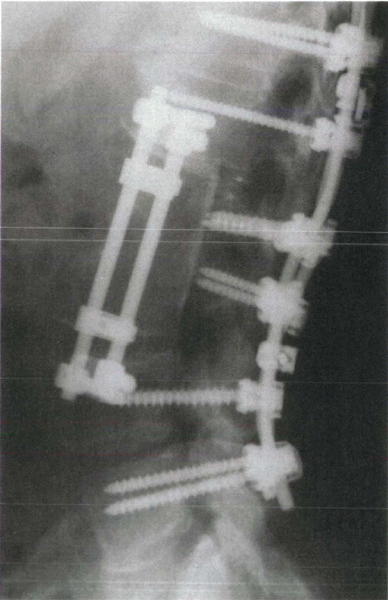 |
|
Figure 8-5
Plain radiograph after anterior débridement, anterior strut grafting (femoral allograft) with instrumentation, and posterior fusion with instrumentation. This treatment was performed for multisegmental pyogenic osteomyelitis. |
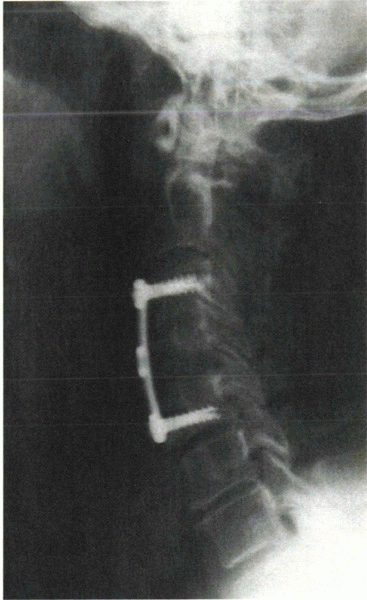 |
|
Figure 8-6
Plain radiograph after anterior cervical débridement and fusion with anterior instrumentation only. This was performed for the infection seen in Figure 8-4. |
the drug regimens discussed in the nonoperative treatment section.
After decompression of an epidural abscess without bone involvement,
parenteral antibiotics may be administered for only 2 weeks after
surgery. The proper postoperative antibiotic regimens should be based
on isolation and sensitivity results and sequential laboratory markers
(ESR and CRP). Patients with a history of intravenous drug abuse should
be covered empirically for gram-negative infection.
specific surgical management. Patients who undergo anterior
decompression and fusion without instrumentation should be protected
with 3 months of external stabilization, whereas patients with combined
anterior/posterior procedures may not require long-term bracing or
casting. Despite these general guidelines, surgeon preference is the
rule when determining postoperative immobilization parameters. Despite
these differences, most surgeons recommend early and progressive
mobilization to prevent bed rest-associated morbidity.
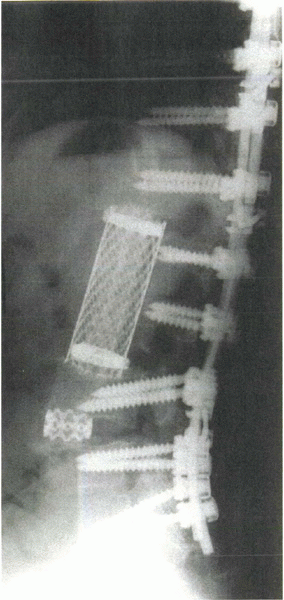 |
|
Figure 8-7
Postoperative radiograph shows the use of metal cage instrumentation anteriorly after débridement of infection in these areas. Autograft or allograft bone can be used to fill and supplement the cages. |
decompression procedures should be thoroughly débrided and irrigated.
Hardware most often is left in place, but exchanging metal rods or
loose screws can be done. Suction drains should be placed or the wound
should be packed open. Serial débridements may be necessary for
appropriate treatment, and occasionally tissue flaps or tissue
expanders are employed for adequate hardware and wound coverage.
S, Schlegel U, Printzen G, et al. Influence of materials for fixation
implants on local infection: an experimental study of steel versus
titanium DCP in rabbits. J Bone Joint Surg Br 1996;19:109-116.
JM, Kersley JB. Pyogenic non-tuberculous spinal infection: an analysis
of thirty cases. J Bone Joint Surg Br 1979;61:47-55.
F, Bohlman H, Soni P, et al. Pyogenic and fungal vertebral
osteomyelitis with paralysis. J Bone Joint Surg Am 1983;65:19-29.
MF, Cooper PR, Errico TJ. Posterior plates in the management of
cervical instability: long term results in 44 patients. J Neurosurg
1994;81:341-349.
JF. Anatomic basis for the pathogenesis and radiologic features of
vertebral osteomyelitis and its differentiation from childhood
discitis. Acta Radiol Diag 1985;26:137-143.
P, Rigamonti D. Spinal epidural abscesses: a review of epidemiology,
diagnosis, and treatment. J Spinal Disord 1999;12:89-93.
HJ, Lin HJ, Liu YC, et al. Spinal epidural abscess—experience with 46
patients and evaluation of prognostic factors. J Infect 2002;45:76-81.
