Nerve Injuries
interpreter of, our external environment. This system, along with the
optic nervous system, makes it possible for us to not only survive, but
to flourish in our environment.
of acute nerve injuries as seen in the hand, wrist, and forearm, and
will emphasize the diagnosis of acute nerve injuries and the management
of commonly encountered injuries of the three major upper extremity
nerves and their branches.
deformities, and findings seen following nerve injuries, repair
techniques, and some of the tendon transfers used to treat irreparable
nerve injuries.
These figures represent the most usual or common arrangement of the
nerves, and do not depict anatomic variations. The distribution of the
cutaneous nerves in the upper extremity given in Figure 13-2 and Figure 13-3 indicates the most common distribution of the cutaneous branches of the three major nerves in the hand.
autonomic fibers. The motor fibers originate from the motor neurons in
the anterior horn of the spinal cord. The sensory fibers originate from
the sensory neurons in the dorsal root ganglia. The autonomic fibers
are either preganglionic, arising from the neurons in the
brainstem/spinal cord, or postganglionic, arising from the neurons in
paravertebral ganglia.
localization in the peripheral nerve trunk that correlates to distal
function. Motor neurons serving a single muscle are found grouped
together within the anterior horn of the spinal cord.
macroscopic anatomy of a typical peripheral nerve as seen in the upper
extremity. The following descriptive terms are useful anatomic points
of understanding about nerve injury and repair.
that represents the outermost layer of the nerve as well as an internal
investing layer that surrounds fascicles and groups of fascicles. The
epineurium is an elongation of the dural sleeve of the spinal nerve
roots, and is divided into external and internal layers. The external
epineurium surrounds the peripheral nerve, and anchors blood vessels
entering from the surrounding tissue. The internal epineurium is
present between fascicles, and cushions them from external force. It
also contains blood vessels. The percentage of nerve cross-sectional
area represented by the epineurium may vary, and greater amounts may be
found in the nerves in the region of joints and in more exposed
locations. This layer may thicken or enlarge in response to trauma, and
may represent a large portion of the scar seen after nerve injury. The
epineurium is surrounded by a loose collection of areolar tissue that
under normal circumstances (absence of extrinsic scar tissue) accounts
for the longitudinal excursion of the nerve and the avoidance of
traction injuries.
composed of up to 10 concentric lamellae of flattened cells, with
prominent basement membranes that are linked tightly together.
Longitudinally and obliquely oriented collagen fibers are present
between these lamellae. The perineurium functions as an extension of
the blood-brain barrier, controlling the intraneural ionic environment
by limiting diffusion, blocking the spread of infection to the
endoneurium, and maintaining a slightly positive intrafascicular
pressure. The perineurium surrounds each fascicle
and
provides a diffusion barrier. This layer maintains a positive pressure
gradient that is manifested by the bulging fascicles of a freshly cut
nerve.
 |
|
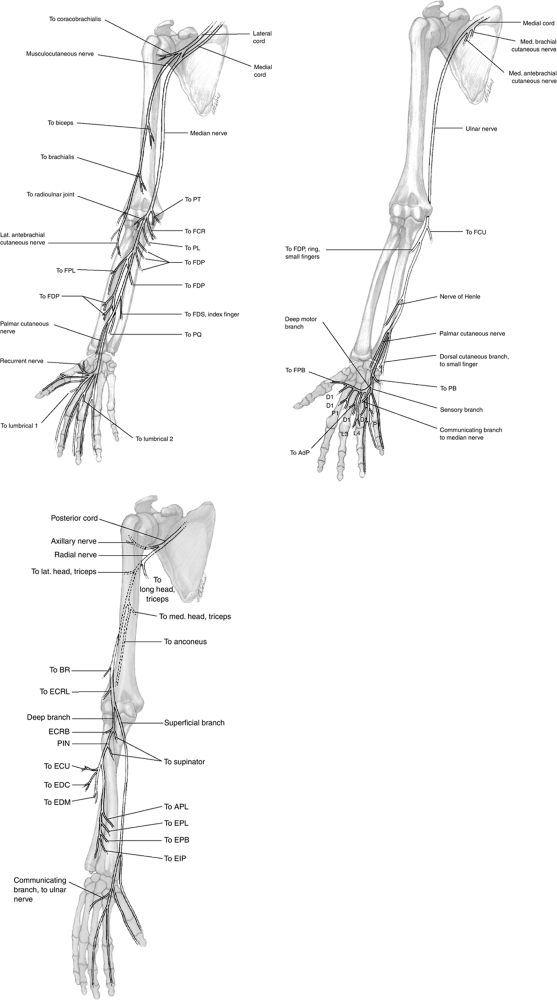
Figure 13-1
The configuration and anatomic relationships of the three major nerves of the upper extremity, and their branches showing distribution and muscles innervated. |
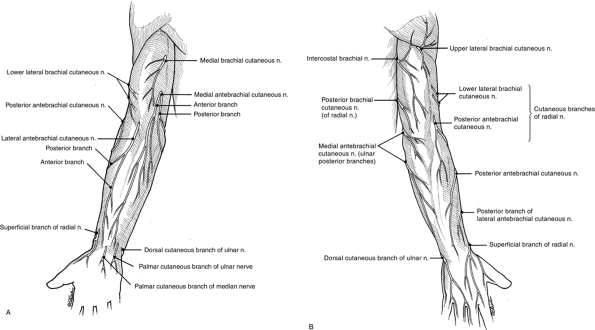 |
|
Figure 13-2 The cutaneous nerves of the upper extremity of the (A) anterior and (B) posterior aspects of the upper extremity.
|
“packing” among axons within the perineurium. The endoneurium
participates in formation of the Schwann cell tube that contains the
myelinated axon and its associated Schwann cells. Schwann cells form
membranous expansions around axons, and form the myelin sheath that, in
oversimplified terms, represents the “insulation” around the axon and
promotes efficient conduction and velocity of action potentials. Large
myelinated axons have two layers of collagen (endoneurium); the outer
is longitudinally oriented and the inner is arranged randomly and is
associated with reticulin. Small myelinated axons have only the outer,
longitudinal layer. The Schwann cell basement membrane forms the inner
lining of this tubular structure. Endoneurial collagen resists
longitudinal stress, and the Schwann cell participates in a complex
homeostatic relationship with the axon.
can be manipulated surgically; it contains axons enclosed by
endoneurium and is in turn wrapped in perineurium (Figure 13-4).
The number of fascicles in a given nerve may vary in number and size.
Interconnections may occur between fascicles. Fascicular group mapping
has permitted more accurate reapproximation of nerves, and
identification of these fascicular groups as to size and axial
orientation may allow the surgeon to more appropriately reconnect or
join severed nerves either primarily or with nerve grafts.
nutrient vessels join a plexus of predominantly longitudinal vessels in
the epineurium. These, in turn, supply a second plexus that lies among
the lamellae of the perineurium. Perineurial vessels may travel for
long distances before entering the endoneurium. This longitudinal
plexus of blood vessels allows a nerve to be mobilized for a
significant distance without ischemic compromise.
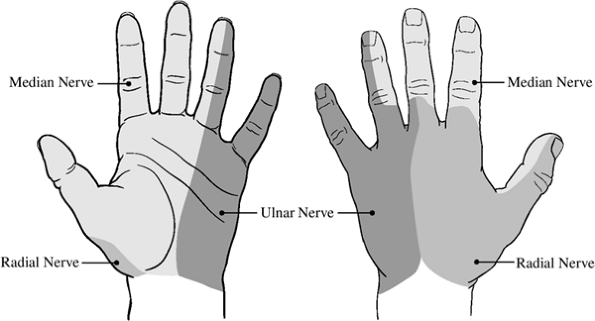 |
|
Figure 13-3 The terminal (cutaneous) distribution of the median, ulnar, and radial nerves in the palm and back of the hand.
|
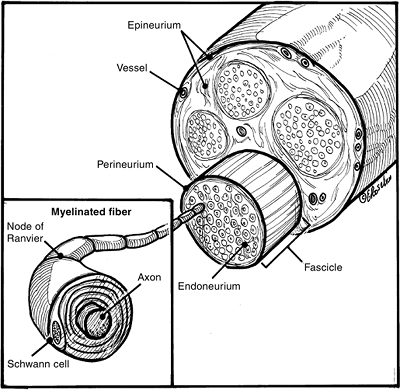 |
|
Figure 13-4
Typical cross-sectional anatomy of a peripheral nerve showing the nerve axons surrounded by the investing layers of endoneurium, perineurium, and epineurium. Note the grossly visible vascular network in the perineurium (see text for details). |
unit of the peripheral nerve. Each neuron consists of a cell body with
cytoplasmic extensions, called dendrites, and an axon (Figure 13-5).
nucleoli, ribosomes, endoplasmic reticulum, and the Golgi apparatus. It
synthesizes structural components of the neuron.
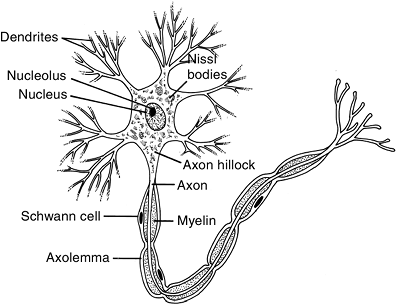 |
|
Figure 13-5
The basic functional unit of the peripheral nerve is the neuron, which consists of a cell body, an axon, and dendrites. The axon and dendrites are protoplasmic extensions of the cell body. Some axons are unmyelinated and some are myelinated. |
the neuron at a confluence of cytoplasm called the axonal hillock. The
axon contains axoplasm, microtubules, and neurofilaments surrounded by
the axolemma, which serves as the surface membrane. The axon functions
as a means of bidirectional axonal transport and nerve conduction.
the larger axons have a myelin sheath. In larger myelinated axons,
Schwann cells are present along the entire length, and are said to be
the glial cells of the peripheral nervous system. The myelinated axon
has characteristic indentations at uniform intervals; these are called
the nodes of Ranvier and are myelin-free
zones that facilitate ionic exchange and propagation of electric
conduction. The diameter of the axon is one factor that may determine
the presence or absence of myelin. The size of the axon may determine
the thickness of the myelin layer. Motor fibers have thicker myelin
sheaths than sensory fibers.
several small axons, and in a myelinated nerve, Schwann cells surround
a single axon. The myelin sheath is a complex proteophospholipid that
promotes conduction efficiency and the velocity of action potentials.
Myelinated axons have four distinctive regions: the node of Ranvier, paranode, juxtaparanode, and internode.
Each of these zones is characterized by a specific set of axonal
proteins. Voltage-gated sodium channels are clustered at the nodes,
whereas potassium channels are concentrated at juxtaparanodal regions.
around the sweat duct. This complex consists of several receptors
served by the branches of a single axon.
and is very sensitive to the perpendicular indentation of the skin. The
Merkel cell has a well-circumscribed receptive field of 2 to 4 mm, and
responds to a small area of skin pressure, such as static 2-point discrimination.
dermis, and are most highly developed in the finger pulp or pads. They
are egg-shaped lamellar structures situated closely below the dermis at
the sides of the intermediate ridge, and are anchored by thin strands
of connective tissue.
at the end, and respond to flutter vibration at 30 cps. The Meissner
corpuscle is well equipped for analysis of motion, such as moving 2-point discrimination.
of the palmar and plantar surfaces, and is visible to the naked eye; it
reaches up to 2 mm in length.
|
Table 13-1 Comparison of Sunderland and Seddon Classification of Nerve Injuries
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
devised a classification system of nerve injury based on degree of
disruption of the internal structures of the peripheral nerve. These
systems are simple and widely used today because of their correlation
to the degree of nerve injury and thus provide a useful basis for
comparison of results with various treatments and lend themselves, to
some degree, as prognostic factors following nerve injuries. Sunderland
noted that nerve injuries may be represented by more than one degree of
injury, and that a nerve injury may not always fall into a single
category. Table 13-1 compares these two classification systems
-
Signals from the periphery turn on the
genes in the neuron for successful reinnervation. The neuron receives
specific signals from the axonal injury site, which in turn prime the
cell to initiate the process of degeneration and subsequent
regeneration. -
The first phase
is characterized by the arrival of action potentials generated at the
injury site. This burst of action potentials opens gated calcium
channels, causing an influx of calcium ions that activate
calcium-sensitive protein kinases. These kinases regulate various
transcription factors. -
In the second phase,
protein kinases and other intrinsic constituents activated at the
injury site are transported retrograde, promoting synthesis of proteins
required for
P.216regeneration. Growth-specific proteins are synthesized, and are transported distally.
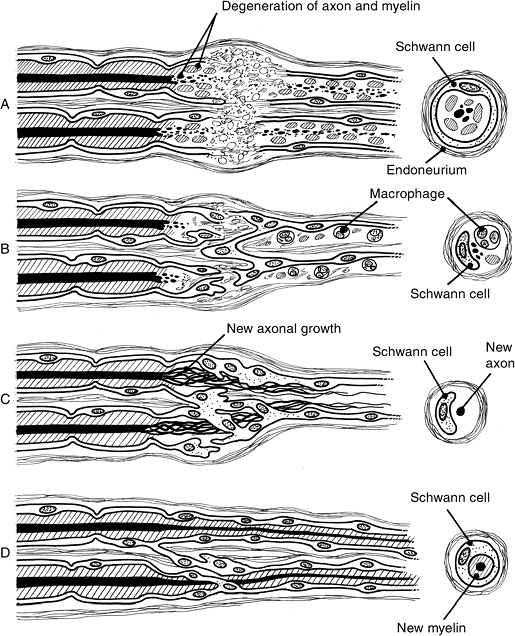
![]() Figure 13-6 Wallerian degeneration and regeneration. A. Breakdown of axoplasm and cytoskeletons into granular, ovoid particles. B. Macrophages appear, and in 2 to 3 days they phagocytize and clear away these particles. C. Schwann cells proliferate by mitosis, and form a line known as Bunger’s band. D.
Figure 13-6 Wallerian degeneration and regeneration. A. Breakdown of axoplasm and cytoskeletons into granular, ovoid particles. B. Macrophages appear, and in 2 to 3 days they phagocytize and clear away these particles. C. Schwann cells proliferate by mitosis, and form a line known as Bunger’s band. D.
Schwann cells wrap around and myelinate newly regenerating axons.
(After Chiu DTW, Choi M, Dellon AL. Nerve Physiology and Repair. In:
Trumble TE, ed. Hand surgery update 3: hand, elbow & shoulder.
Rosemont, IL: American Society for Surgery of the Hand, 2003:287–297). -
The third phase
is mediated by neurotrophic factors, growth-promoting surface
molecules, and cytokines released by the extrinsic cells, such as the
macrophages and Schwann cells at the injury site. These are then taken
up by the injured axon and transported back to the neuron. -
The last phase marks the end of regeneration. Growth factors produced by the target organ arrive.
begin to develop from the most distal node of Ranvier proximal to the
site of injury. The sprout formation site may be close to the injury
site (in a sharp transection) or farther away (in an
avulsion/crush-type injury). Terminal sprouts arise from the tip of the surviving axon.
-
Axoplasm and cytoskeletons break down.
-
Macrophages appear in the injured area and begin to phagocytize the breakdown material.
-
Schwann cells proliferate by mitosis, forming a line known as Bunger’s band.
-
Schwann cells envelope and form myelin around new regenerating axons.
immediately following an injury. Circulating macrophages and those
within the nerve are attracted to the injured area in large numbers.
After 2 or 3 days, they start to phagocytize these particles and clear
them away.
line known as Bunger’s band. This degenerative process takes at least 1
to 2 weeks, and electrodiagnostic tests performed during this period
may not pick up any abnormality.
macrophages, Schwann cells, various neurotrophic factors, the injured
neuron, and end organs. They influence each
other in an intricate interplay, which is currently only partially understood.
distal endoneurial tube may collapse, rendering regeneration of the
axon difficult, if not impossible.
reinnervated following a delayed surgical nerve repair, rather than due
to degeneration of muscle fibers and motor end plates, as previously
thought.
interdependent. This relationship is mediated by both neurotrophic
factors and neurite outgrowth promoting factors.
communication between neurons and Schwann cells. Neurite outgrowth
promoting factors facilitate attachment of growing axons to other axons
and/or Schwann cells, and thus guide nerve regeneration.
which have been identified: neurotrophins, neurokines, and the
transforming growth factor (TGF).
and neuromuscular transmission are beyond the scope of this text. For
more details of these complex processes, refer to the Suggested
Readings—particularly the chapter on Nerve Physiology and Repair in Hand Surgery Update.
-
Peripheral nerves are most always mixed nerves (they contain both motor and sensory nerve fibers), but the injury may not always involve both components of the nerve.
-
Nerve injuries may be partial, and may spare some vital nerve pathways while injuring others.
-
The appraisal of peripheral nerve injuries involves the examination of both sensory and motor components of the nerve that is being evaluated.
-
Sensory evaluation is based on examination of a nerve’s autonomous zone of innervation (the anatomic site least likely to have any overlap with another nerve).
-
Motor evaluation is focused on the
functional motor unit that is the last or most terminal muscle to be
innervated by the nerve under evaluation, or sometimes by the ease of
examination of a muscle known to be innervated by that nerve. -
In an acute situation in the emergency room, the sensory evaluation of an injured nerve is best determined by light touch
rather than by a pin or needle (especially in children). Two-point
discrimination is a valuable tool for evaluation of sensation, but it
may be difficult to achieve in an acute injury in the emergency room. -
Both sensory and motor evaluations are made before any form of local anesthesia is given.
-
In most, if not all instances, the
diagnosis of a peripheral nerve injury may be made without
inappropriate exploration of the wound in an emergency room setting.
The diagnosis is made based on knowledge of the anatomy involved and on
a focused examination that utilizes that knowledge. -
When in doubt as to the extent or nature
of injuries to the nerve from lacerations, explore the wound under
appropriate anesthesia in an operating room.
-
This is a clinical sign that’s useful for
determining the level of nerve injury or recovery based on the presence
of injured or regenerating axons. -
The test is performed by digital
percussion of the nerve distal to the site of nerve injury, with
proximal advancement of the percussion until the site of injury is
reached or the zone of regeneration is identified. -
The percussion, performed gently with the
examiner’s fingertip, progresses along the course of the nerve until
the endpoint is identified by paresthesia localized either to the zone
of injury in cases of complete nerve interruption, or distally into the
zone of cutaneous innervation. -
Some clinicians prefer to percuss from proximal to distal to the site of the lesion downward until the paresthesia disappears.
-
The paresthesia, or “pins and needles”
sensation, is caused by the regenerating axons that are sensitive to
percussion, in part because of the loss of the myelin sheath as part of
Wallerian degeneration. -
A positive response over a long segment of nerve may be due to unequal growth rates of various sensory fibers.
-
The sign may be absent in the first 4 to
6 weeks after nerve suture, and the onset and progress of the sign may
be inversely proportional to the severity of the injury. -
Steady distal progression of the endpoint is a favorable sign in nerve regeneration, but it must be recognized that the sign is qualitative, not quantitative.
-
A nerve laceration is said to be high or low (proximal or distal).
-
More than one nerve may be injured in a combined injury. An example is a median and ulnar nerve laceration at the wrist.
-
Combined injuries are often associated
with injuries to the vascular supply, which may complicate treatment
and recovery. Furthermore, in some unusual instances, the injured
nerves may be at different anatomic levels.
-
Figures 13-1 and 13-3 show the classic patterns of motor innervation and sensory distribution of the specific nerves.
-
The three major nerves may be injured at
any level, but our discussion is confined to nerve injuries in the
forearm, wrist, or hand. -
The loss of motor function and sensation will depend on the level of injury.
-
Lesions may be represented by contusion, crush, or partial or complete lacerations.
-
Our discussion will assume that the injuries are complete, and that motor and sensory components are completely lacerated.
-
Sensory deficit in the flexor aspect of the thumb, index finger, middle finger, and radial half of the ring finger.
-
The autonomous zone of deficit evaluation is the flexor aspect of the terminal phalanx of the index finger (Figure 13-7).
-
Loss of pronation of the forearm, flexion
at the interphalangeal (IP) joint of the thumb, distal interphalangeal
(DIP) joints of the index and middle fingers, proximal interphalangeal
(PIP) joints of the four fingers, and abduction-opposition of the thumb.
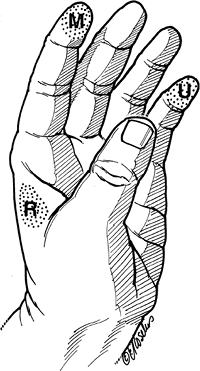 |
|
Figure 13-7
The three autonomous zones of sensation in the hand. These are the areas least likely to have any sensory nerve overlap, and thus may be considered reliable areas to test for sensation of the respective nerves involved. M, median, flexor aspect of the terminal phalanx of the index finger; R, radial, web space between the thumb and index finger; U, ulnar, flexor aspect of the terminal phalanx of the little finger. |
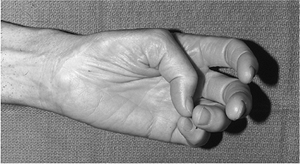 |
|
Figure 13-8 Several week old median nerve laceration showing loss of opposition of thumb, and atrophy of the thenar muscles.
|
-
Ulnar deviation of wrist with wrist flexion.
-
There is also an inability to perform pulp-to-pulp contact between the thumb and the adjacent digits (opposition).
-
The thumb stays adducted in the palm during attempted opposition (Figure 13-8).
-
Sensory deficit in the flexor aspect of the thumb, and index finger, middle finger, and ulnar half of the ring finger.
-
The autonomous zone of deficit is the flexor aspect of terminal phalanx of the index finger (see Figure 13-7).
-
Loss of abduction-opposition of thumb.
-
Isolated injury of the motor branch of
the median nerve will result in loss of thumb abduction-opposition
without sensory deficit (Figure 13-8).
-
Loss of sensation in the flexor/dorsal
aspect of the little finger, ulnar half of the ring finger, and
flexor/dorsal aspect of ulnar half of the hand. -
The autonomous zone of deficit is the flexor aspect of the terminal phalanx of the little finger (Figure 13-7).
-
Loss of flexion of the flexor digitorum
profundus (FDP) of the ring and little fingers; loss of function in all
intrinsic muscles except for the lumbricals of the index and middle
fingers; and loss of flexion in the flexor carpi ulnaris (FCU). -
Profound wasting of the intrinsic muscles is noted (Figure 13-9).
-
Wrist flexion is associated with slight radial deviation.
-
Attempts to extend the ring and little fingers result in hyperextension at the metacarpophalangeal (MCP) joints,
P.219and in flexion at the PIP joints. This is called Duchenne’s sign (Figure 13-10).
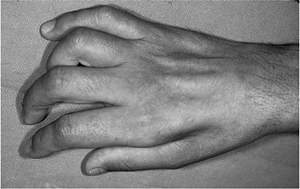 Figure 13-9 High ulnar nerve laceration showing profound atrophy of the intrinsic muscles of the hand.
Figure 13-9 High ulnar nerve laceration showing profound atrophy of the intrinsic muscles of the hand. -
Pressure or force over the proximal
phalanges of these digits by the examiner promotes extension of the PIP
joints; this is called Bouvier’s sign or maneuver (Figure 13-11). -
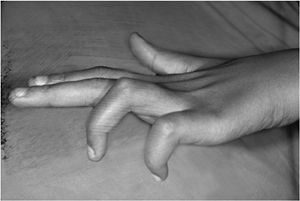
A weak and unstable pinch, with hyperextension of the thumb MCP joint (Jeanne’s sign) and with hyperflexion of IP joint (Froment’s sign), are also classic signs of ulnar nerve palsy (Figure 13-12).
-
There is an inability to cross the middle
finger over the index finger or to cross the index over the middle
finger. This indicates a loss of function in the second and first
dorsal interosseous muscles, respectively.![]() Figure 13-10
Figure 13-10
Note the claw deformity of the ring and little fingers in this patient
with a low ulnar nerve lesion. This deformity is characterized by
hyperextension of the ring and little finger MCP joints and flexion of
the PIP joints, and is called Duchenne’s sign.
The deformity is due to loss of the ulnar-nerve-innervated lumbricals
and the dorsal and palmar interosseous muscles that normally flex the
MCP joints and extend the PIP joints, and thus “balance” the effects of
the extrinsic flexors of the fingers.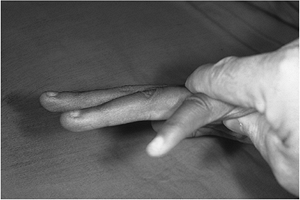 Figure 13-11
Figure 13-11
Pressure or downward force over the proximal phalanges of the clawed
ring and little fingers (low ulnar nerve palsy) reproduces the action
of the intrinsic muscles and corrects most of the claw deformity. This
finding is called Bouvier’s maneuver. -
The claw deformity may not be as
prominent in high ulnar lesions compared to low lesions since both the
flexor digitorum superficialis (FDS) and the FDP extrinsic finger
flexors are intact in low lesions, and since they account for a
stronger flexion deformity in the ring and little fingers.
-
Loss of sensation in the flexor/dorsal
aspect of the little finger, ulnar half of the ring finger, and
flexor/dorsal aspect of the ulnar half of the hand. -
The autonomous zone of deficit is the flexor aspect of the terminal phalanx of the little finger.
 |
|
Figure 13-12
Froment’s and Jeanne’s signs as seen in ulnar nerve palsy in the left hand. Note the compensatory hyperflexion of the IP joint (Froment’s sign) and hyperextension of the MCP joint of the thumb (Jeanne’s sign) during pinch. This posture is due to loss of the stabilizing force of the adductor muscle on the MCP joint of the thumb and compensatory hyperflexion of the thumb IP joint. Note also the atrophy of the first dorsal interosseous muscle in the thumb-index web space. |
 |
|
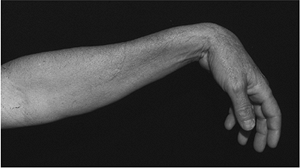
Figure 13-13
This patient demonstrates a high radial nerve palsy. Note the loss of extension of the wrist, thumb, and fingers secondary to a laceration of the radial nerve above the elbow. |
-
Motor deficits are the same as in high
ulnar nerve lesions, except that the FDP function to ring and little
fingers is intact, and may accentuate the claw deformity.
-
The dynamic posture is the same as in high ulnar nerve lesions.
-
There is loss of sensation over the dorsal radial aspect of the hand and thumb.
-
The autonomous zone is in the web space between the thumb and index finger.
-
There is loss of extension of the wrist, fingers, and thumb mediated by the EDC, EDM, EIP, ECRB, ECRL, ECU, EPL, EPB and APL.
-
A high radial nerve lesion results in the loss of wrist, finger, and thumb extension, and is commonly called a “wrist drop” (Figure 13-13).
-
This represents a total and high radial nerve lesion before its division into the motor and sensory components.
-
This division may occur in a region that
is 2.5 cm above or 3.0 cm below a line drawn in the coronal plane
between the medial and lateral epicondyle of the humerus (Hueter’s
line).
-
The high and low lesion convention has
worked well for the median and ulnar nerves, but works less well for
the radial nerve because the motor and sensory components are widely
separated in the proximal forearm. -
The sensory component
of the radial nerve may be injured in a “high or low” anatomic
position. This results in the same clinical manifestations that are
noted for the high lesion.
-
The motor deficit will vary according to the level of injury and the sequence or order of innervation of the respective muscles.
-
The sequence of innervation order is quite variable, but Figure 13-1 shows a common one.
-
A common sequence is the innervation of the ECRL, supinator, and ECRB, in that order.
-
The EDC is most always innervated before the EIP, APL, and EPL.
-
The EDM is usually innervated before the EIP, and the APL before the EPL.
-
-
Primary repair is defined as repair within hours of injury.
-
Delayed primary repair is repair performed 5 to 7 days after injury.
-
A repair performed more than 1 week after injury is called a secondary repair.
-
Primary nerve repair has emerged as the
treatment of choice when conditions permit (Box 13-1). If these
conditions cannot be met, it is better to delay repair. -
Secondary repair under favorable
conditions is usually associated with improved outcomes compared to
nerve repair done under less than ideal circumstances. -
In cases where timely repair cannot be
performed, the nerve ends should be sutured together to prevent
retraction. This will facilitate a future definitive repair.
-
Sharp transections without any element of crush injury
-
Absence of significant tension at the repair site when the ends are joined
-
Minimal wound contamination; a suitable bed of viable muscle, fat, or tenosynovium
-
Absence of other injuries that preclude timely restoration of circulation, skeletal stability, or soft tissue cover
-
A suitable operating room and equipment, including magnification
-
A patient in suitable metabolic and emotional condition to undergo surgery
-
An intoxicated patient or a patient who has just attempted suicide is not an ideal candidate for primary repair.
-
-
Magnification improves the results of nerve suture and the operating microscope is a useful tool to facilitate nerve suture.
-
Placement of the proximal and distal nerve stumps on suitable background material in a bloodless field aids in visualization.
-
Correct rotational orientation is
obtained by matching the fascicular bundles with respect to size and
position, and matching the orientation of the vascular landmarks. -
The cut ends are trimmed perpendicular to
their long axis to remove any damaged tissues and to form a uniform
surface for contact between the ends. -
A smooth and uniform transection prior to
repair may be achieved by devices that hold or stabilize the nerve
during the transection. -
A single suture is placed to join the epineurial edges furthest from the surgeon.
-
A second suture is then placed at 180 degrees from the first to form equal halves.
-
Done properly, this will result in
matching opposing surfaces, without bulging, retraction, or twisting of
the individual fascicles. -
An 8-0 or 9-0 suture is used for these
two critical stitches in a major peripheral nerve, and the tails are
left long to permit rotation of the nerve. -
The inability to bring the nerves ends
together with 8-0 sutures indicates excessive tension, and further
mobilization or grafting should be considered. -
Additional interrupted sutures of 10-0 are used to bisect the 180-degree arc formed by the first two sutures.
-
Additional sutures are added in a similar
fashion to complete the repair but a “watertight” repair is neither
necessary nor desired.
-
Group fascicular suture requires greater magnification and is technically more challenging than epineurial suture.
-
This suture technique is less tolerant of repair under tension due to the less substantial nature of the internal epineurium and perineurium.
-
The proximal and distal stump surfaces
are inspected to identify fascicular groups. Fascicular groups tend to
separate from other fascicular groups, and with gentle traction and
dissection with curved microscissors, fascicular groups may be
identified. -
Matching fascicular groups are trimmed at
right angles to their longitudinal axis and separated for a distance of
3 to 4 mm in the proximal and distal stumps. -
A retention suture placed in the
posterior epineurium is useful to control tension and permit rotation
of the nerve to aid in placement of sutures in the various fascicular
groups. -
The fascicular groups are joined from
back to front by sutures placed in the internal epineurium or
perineurium. Only the minimum number of sutures necessary to produce
uniform coaptation of the fascicular group is placed.
-
The separation of individual fascicles is technically challenging and is less commonly used for nerve suture.
-
Large fascicles may accept two 10-0 sutures, but a single suture may be appropriate for smaller fascicles.
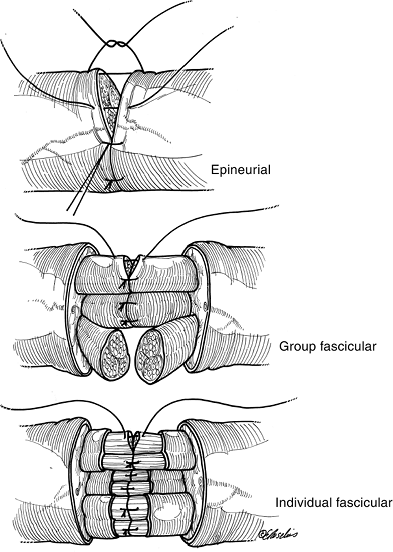
![]() Figure 13-14 The three techniques of peripheral nerve repair.
Figure 13-14 The three techniques of peripheral nerve repair. -
This technique may be most useful in partial nerve lacerations where only a portion of a group fascicle has been lacerated.
-
Figure 13-14 demonstrates the three techniques of peripheral nerve repair.
-
Accurate matching of sensory and motor components of a severed nerve is an important factor in the outcome of nerve repair.
-
Techniques that aid in matching include
using intraneural mapping, intraoperative nerve stimulation, and
histochemical identification of the motor and sensory components. -
The later two techniques have time
constraints and technical aspects in their performance that limits
their use. They may be most useful in secondary nerve reconstruction.
-
There are many instances in nerve repair where loss of nerve substance does not permit a tension free rejoining of the injured nerve.P.222
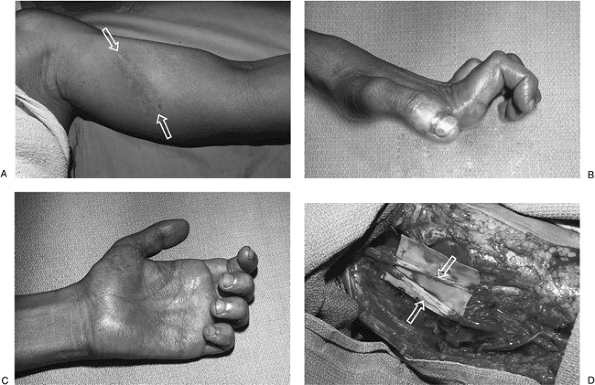 Figure 13-15 A. This 22-year-old man sustained a deep laceration to the inner aspect of his left arm. B, C.
Figure 13-15 A. This 22-year-old man sustained a deep laceration to the inner aspect of his left arm. B, C.
This resulted in sensory deficits in the median and ulnar distribution
and a median and ulnar claw hand (a combined high median and ulnar
palsy). D. He was treated with fascicular
bundle nerve grafts (sural nerves) to the median and ulnar nerves, with
recovery of wrist and finger flexion and protective sensation in the
hand.-
This is most often seen in secondary
nerve repair where excision of the scarred and damaged nerve ends
results in a significant gap that mitigates against a tension-free
union.
-
-
Earlier efforts at nerve graft included
interposition of trunk grafts that matched the size of the injured
nerve; cable grafts composed of smaller nerves, bundled together, to
fill the gap; and a pedicle nerve graft in which one major nerve was
sacrificed and used to replace what was considered to be a more
important nerve. -
The current technique to restore continuity in a nerve with a significant gap is called group fascicular nerve grafting.
-
The technique consists of removing all
interposed scar tissue between the two ends of the involved nerve, and
interposing autogenous nerve grafts (usually the sural nerve or nerves)
to matching fascicles in the proximal and distal stumps. -
Matching of fascicles is most easily
achieved in gaps of 4 to 6 cm, although larger gaps may be filled and
suitable results obtained (Figure 13-15). -
The lateral antebrachial cutaneous nerve in the forearm may be used to fill gaps in digital nerves.
grafts, nerve conduits, and neurotropism is beyond the scope of this
chapter.
-
In general terms, the results following
nerve repair are best within the first 3 months after injury—but
reinnervation may be expected within 1 year following injury. -
No reinnervation can be expected after 3 years.
-
In some instances, nerve repair is not possible—but useful upper extremity function may be restored by tendon transfers.
-
In some instances following nerve repair, a useful level of sensory recovery (protective sensation or better) may be anticipated without motor recovery. It is in these cases that tendon transfer may be most useful.
-
Tendon transfers work best in supple
joints without fixed contracture and when suitable soft tissue beds are
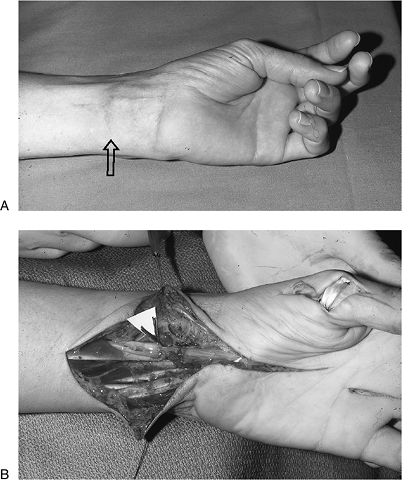
available for passage of the tendons.P.223![]() Figure 13-16 A.
Figure 13-16 A.
Wrist scar, loss of opposition, and thenar eminence atrophy can be seen
in this 65-year-old male with complete sensory loss in the median nerve
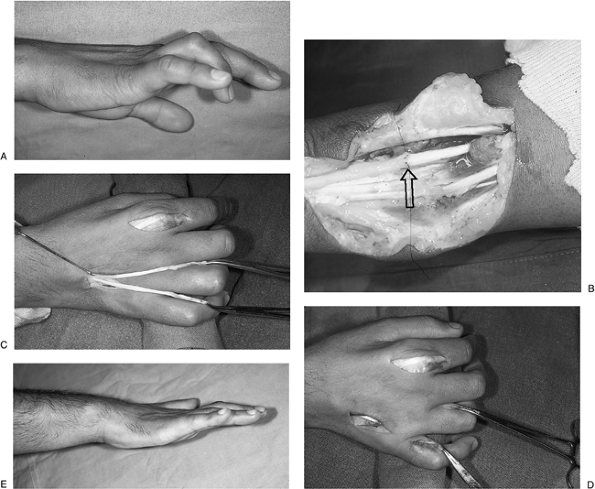
distribution. B. Epineurial repair of the median nerve was performed along with a FDS opponensplasty.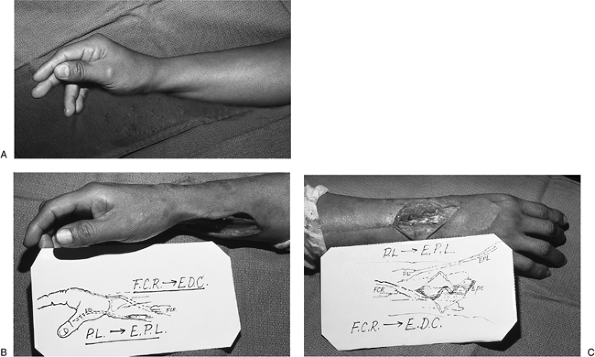 Figure 13-17 Intermediate level radial nerve palsy. A.
Figure 13-17 Intermediate level radial nerve palsy. A.
Preoperative appearance of an injury to the posterior interosseous
branch of the radial nerve (PIN) with loss of thumb and finger
extension. B, C. Function was restored by transfer of the PL to the rerouted EPL and transfer of the FCR to the EDC tendons.P.224![]() Figure 13-18 A. Clinical appearance of an ulnar claw hand. B. Secondary epineurial repair of the ulnar nerve was performed. C, D.
Figure 13-18 A. Clinical appearance of an ulnar claw hand. B. Secondary epineurial repair of the ulnar nerve was performed. C, D.
The EIP tendon was split into two tails, passed through the
interosseous spaces beneath the axis of rotation of the MCP joints, and
sutured to the radial lateral bands of the ring and little fingers. E.
This transfer replaced the function of the interosseous muscles,
rebalanced the extrinsic flexors and extensors, and corrected the claw
deformity. -
The simple treatment algorithm is: (1)
what function is lost, and (2) what expendable muscle/tendon units are
available for transfer. -
Selection of an appropriate muscle tendon
unit is based on its cross-sectional area (strength) and excursion
(fiber length), in order to mimic as much as possible the muscle
tendon/unit being replaced. -
Mechanical factors include orientation of
the transfer for a straight-line pull, one transfer to perform one
function, appropriate tension, placing the transfer in the proper
relationship to the joint axis, and when needed, a strong and suitably
placed pulley or fulcrum.
transfers used to restore function in the upper extremity. Refer to the
Suggested Reading list for a more comprehensive discussion of the topic.
preoperative appearance of a 65-year-old male with a one-year-old
laceration of the flexor aspect of the left wrist. There was loss of
sensation in the median nerve distribution and loss of opposition of
the thumb. Treatment included excision of the median nerve neuroma,
epineurial repair, and opponensplasty using the FDS of the ring finger
to the abductor position of the thumb. Opposition was restored along
with protective sensation in the median nerve distribution.
with loss of thumb and finger extension. The level of injury was distal
to the innervation of the wrist extensors, so wrist extension was
maintained. Restoration of thumb and finger extension was achieved by
transfer of the PL to the rerouted EPL and transfer of the FCR to the
EDC tendons.
typical claw deformity of the ulnar nerve. It resulted from a
laceration of the ulnar nerve at the distal forearm. Treatment was by
secondary ulnar nerve repair (epineurial) and transfer of a two-tailed
tendon graft using the EIP sutured to the radial side of the lateral
bands in the ring- and little-finger proximal phalanges.
TM. Nerve repair and grafting. In: Green DP, Hotchkiss RN, Pederson WC,
eds. Green’s operative hand surgery. 4th Ed. New York: Churchill
Livingstone, 1999:1381–1403.
DTW, Choi M, Dellon AL. Nerve physiology and repair. In: Trumble TE,
ed. Hand surgery update-3, hand, elbow and shoulder. Rosemont, IL:
American Society for Surgery of the Hand, 2003:287–297.
ME. Internal anatomy of the peripheral nerve. In: Hunter JM, Schneider
LH, Mackin EJ, eds. Tendon and nerve surgery in the hand. St. Louis:
Mosby-Year Book, 1996:19–25.
H, Meissl G, Berger A. Further experience with intrafascicular grafting
of the median, ulnar, and radial nerves. J Bone Joint Surg
1976;58A:209–218.
G. Combined nerve palsies. In: Green DP, Hotchkiss RN, Pederson WC,
eds. Green’s operative hand surgery. 4th Ed. New York: Churchill
Livingstone, 1999:1542–1555.
JE. Tendon transfers. In: Trumble TE, ed. Hand surgery update-3, hand,
elbow and shoulder. Rosemont, IL: American Society for Surgery of the
Hand, 2003:353–370.
KL. Anatomy of the peripheral nerve. In: Hunter JM, Schneider LH,
Mackin EJ, eds. Tendon and nerve surgery in the hand. St. Louis:
Mosby-Year Books, 1996:11–18.