Intervertebral Disc Structure, Composition, and Mechanical Function
IV – Basic Science > 23 – Intervertebral Disc Structure,
Composition, and Mechanical Function
vertebrae connected by flexible intervertebral discs. Because of their
flexibility, the intervertebral discs allow the spine to twist and bend
throughout a wide range of postures. In addition to allowing
flexibility, the intervertebral discs function in both absorbing energy
and distributing loads applied to the spine. The unique structure and
composition of the intervertebral disc allow for a wide array of
mechanical functions to be performed. It is the disruption of this
relationship that leads to intervertebral disc pathology.
20% to 30% of its length. Apart from the fused vertebrae of the sacrum
and coccyx, the only vertebrae not connected by discs are the atlas and
axis, which pivot at the specialized atlanto-axial joint, and the
articulation between the atlas and the base of the skull, which
articulate at the occipitoatlantal joint; this articulation also does
not contain a disc (Fig. 23-1).
-
Non-disc structures also connecting the vertebral bodies (Fig. 23-2)
-
Anterior and posterior longitudinal ligaments
-
Ligamenta flava
-
Interspinous ligaments
-
Supraspinous ligaments
-
-
Shape
-
Roughly cylindrical
-
Most discs appear wedge-shaped because the anterior height is greater than posterior.
-
Cross-sectional area increases almost linearly from the cervical to lumbar segments.
-
Unlike cross-sectional area, disc height does not vary regularly along the length of the spine.
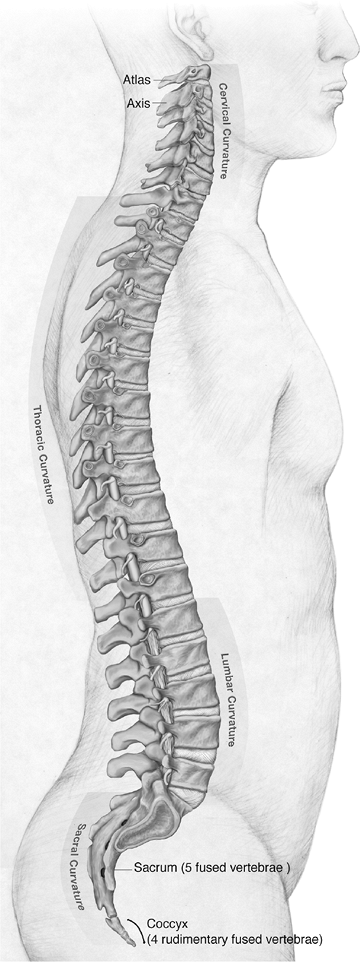 Figure 23-1 Lateral view of the human spine. (Asset provided by Anatomical Chart Co.)
Figure 23-1 Lateral view of the human spine. (Asset provided by Anatomical Chart Co.)
-
-
Components
-
Nucleus pulposus: soft inner region
-
Anulus fibrosus: surrounding tough outer lamellae
-
Consists of 12 concentric coaxial lamellae that form a tube-like structure enclosing the nucleus
-
Arranged into a densely packed outer ring and an inner, larger fibrocartilaginous layer (Fig. 23-3)
-
-
-
Vascularity: largest avascular organ in the body
-
Small blood vessels found on the surface of the outer anulus penetrate 1 to 2 mm at most.
-
Simple diffusion is the most important
mechanism for small-molecule transport into the disc and appears to be
the factor most responsible for limiting cell viability.
-
-
Innervation: poorly innervated
-
Sensory nerves do not penetrate deeper than the outer third of the anulus.
-
Main afferent pathway involves the nerve to the vertebral body: sinuvertebral nerve (recurrent nerve of Luschka) (Fig. 23-4).
-
-
Even though the discs may vary in size, they share the same basic structure and composition.
-
Two main structural components of the intervertebral disc
-
Collagen
-
Accounts for 70% of the dry weight of the anulus and <20% of the nucleus
-
Provides tensile strength
-
-
Proteoglycans
-
Account for a minimal percentage of the anulus but as much as 50% of the nucleus in the pediatric population
-
Provide stiffness, compressive strength, viscoelasticity
-
-
-
-
Collagen arrangement
-
Types I and II collagen fibrils
-
Distributed radially in opposing
concentration gradients, with type II mostly in the nucleus pulposus
and type I most concentrated in the exterior of the anulus-
Anulus: I, II, III, V, VI, IX, X
-
Nucleus pulposus: II, VI, IX, X
-
-
-
Type I collagen provides strength to the
tough lamellar sheets that are anchored into the bone of the adjacent
vertebral bodies (Fig. 23-5).
-
-
Water: contained in the matrix of the disc and contributes approximately 65% to 80% of its total weight
-
Aggrecan is the main proteoglycan in the disc.
-
High density of negatively charged
sulfate and carboxyl groups (cations) on the glycosaminoglycan chains
that attract mainly Na+ (anions), which results in a cumulatively
increased osmotic pressure of 1 to 3 atmospheres in the nucleus -
Pressure gradient enables the disc to continually absorb water.
-
Hydration and swelling continue until it is restricted by the collagen network of the disc.
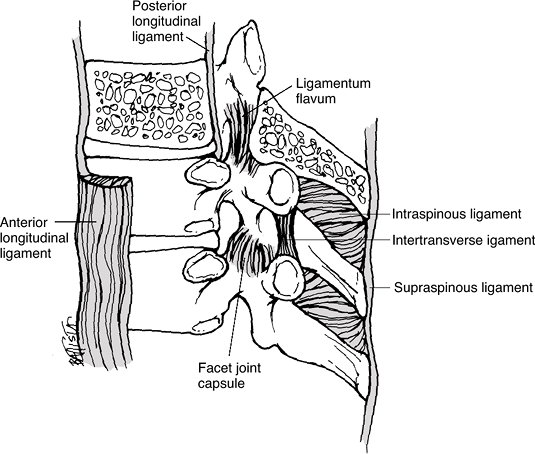
![]() Figure 23-2
Figure 23-2
The ligaments supporting the thoracic spine consist of the capsular
ligaments of the facet joints, the anterior and posterior longitudinal
ligaments, the supraspinous and interspinous ligaments, the ligamentum
flavum, and the intertransverse ligaments. (From Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Baltimore: Lippincott Williams & Wilkins, 2003.) -
Equilibrium between the aggrecan and
collagen provides the load-bearing, compression-resisting tissue that
holds the other units of the spine in correct position while allowing
movement of the spinal column.
P.454 -
-
Withstands the significant forces of an upright posture
-
Forces up to 17,000 Newtons estimated in lumbar discs
-
Functions within a specialized unit called the motion segment (Fig. 23-6), where the basic motions of axial compression, torsional loading, and sagittal and transverse bending or axial torsion occur (Fig. 23-7).
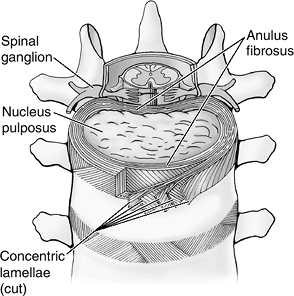
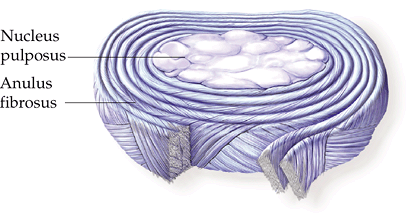 Figure 23-3
Figure 23-3
Structural features of an intervertebral disc. The nucleus pulposus is
the central gelatinous cushioning part of the intervertebral disc
enclosed in several layers of cartilaginous laminae. (Asset provided by
Anatomical Chart Co.)
-
-
Biphasic viscoelastic behavior during loading (Table 23-1)
-
Mechanical loading causes fluid movement within the discs, which gives rise to time-dependent mechanical properties.
-
The less dense inner anulus and nucleus
pulposus undergo larger volumetric changes in response to loads. This
creates flow within the disc that dissipates the energy and causes
viscoelastic creep. -
The stiffer outer anulus converts this compressive load into hoop stresses while the inner layers act as a “shock absorber.”
-
The high tensile modulus of the outer anulus helps to prevent any bulging of the disc from the loads applied.
-
Torques on the motion segment distort the
shape of the anulus without altering the volume, while bending and
compression cause disc bulging, volumetric changes, and endplate
deformation.
-
-
Function of the cartilage endplate
-
The hydraulic permeability causes rapid fluid transport and less pressurization in response to loading.
-
This permeability provides a conduit for
water to flow from and into the disc and thereby helps transfer loads
in a uniform manner across the inner anulus and nucleus pulposus.
-
degenerative disc disease and disc herniation. Changes in volume and
shape are accompanied by gross morphologic and microstructural
alterations (Table 23-2).
The most extensive changes occur after the age of 20 in the nucleus
pulposus, where the number of viable cells and the concentration of
proteoglycans and water decline.
 |
|
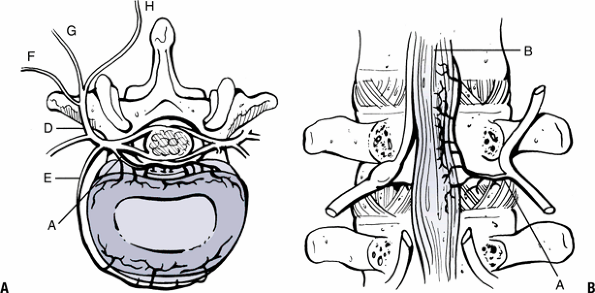
Figure 23-4
The innervation of the disc and facet joints. Sinuvertebral nerve and its branches innervate the dorsal portion of the disc (A) and posterior longitudinal ligament (B). The ventral ramus (C) branches to innervate the ventral disc and anterior longitudinal ligament (E). The dorsal ramus (D) branches into lateral (F), intermediate (G), and medial (H) branches. (From Wetzel FT. Microinnervation: Pain generators. In Bono CM, Garfin SR, eds. Orthopaedic Surgery Essentials: Spine. Philadelphia: Lippincott-Raven, 2004:272–277.) |
complex and poorly understood. It is the result of an intricate
relationship between cellular biology, mechanical factors, and
genetics. Aging and degeneration of discs are separate processes:
although all discs undergo aging, not all of them degenerate. The end
stage of degeneration can be identified by imaging studies and gross
examination, but accepted criteria for the diagnosis and the
distinguishing factors between degeneration and aging have not been
established. It is also unclear as to what degree the degenerative
process contributes to pain. Currently disc degeneration is believed to
be a source of chronic pain, and over 90% of surgical
spine procedures are performed because of consequences of the degenerative process.
 |
|
Figure 23-5
Anterosuperior view of the vertebral column, transversely sectioned through an intervertebral disc. The superficial layers of the anulus fibrosus have been cut and spread apart to show the direction of the fibers. (From Moore KL, Dalley AF. Clinical Oriented Anatomy, 5th ed. Baltimore: Lippincott Williams & Wilkins, 2006.) |
 |
|
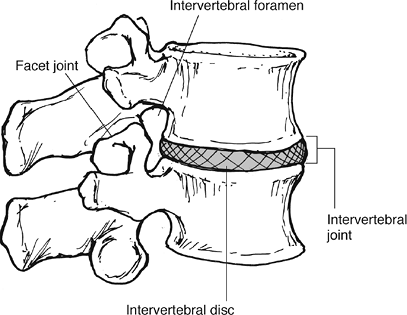
Figure 23-6 A motion segment consists of an intervertebral disc and the two adjacent vertebral bodies. (From Oatis CA. Kinesiology: The Mechanics and Pathomechanics of Human Movement. Baltimore: Lippincott Williams & Wilkins, 2004.)
|
 |
|
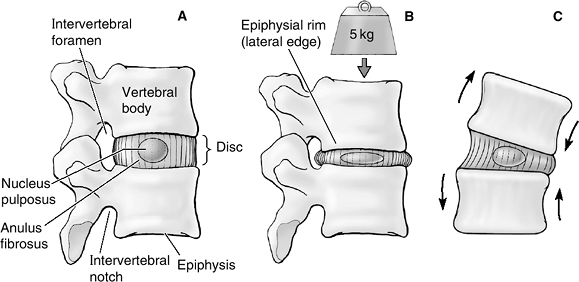
Figure 23-7
(A) The motion segment in cross-section. (B) Under axial compression, the disc bulges. Tensile stresses are generated in the outer anulus and compressive stresses in the nucleus pulposus. High fluid pressures are generated in the nucleus, causing extravasation of fluid. After the cessation of the axial loading, the osmotic pressure causes the extruded fluid to flow back into the disc, restoring its height. (C) Flexion or extension and lateral bending result in eccentric changes of the disc, which results in an alternating suction/extrusion action on the fluids into and out of the disc. (From Moore KL, Dalley AF. Clinical Oriented Anatomy, 5th ed. Baltimore: Lippincott Williams & Wilkins, 2006.) |
-
Degeneration appears to be the natural consequence of two scenarios:
-
Application of normal loads to a disc with abnormal material properties
-
Application of abnormal loads to disc with normal material properties
-
-
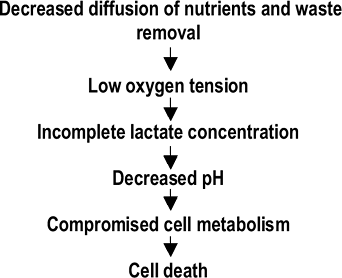
Declining nutritional support is the most important event responsible for the changes in central disc cells and their matrices (Fig. 23-8).
-
Sequence of events resulting in loss of nutritional support
-
Increase in disc volume during growth results in relative decline in the vascular supply.
-
Decreased vascular supply results in
impaired diffusion and convection that is necessary for the transport
of nutrients and removal of waste.
-
-
-
Loss of disc tissue results from the
action of degradative enzymes within the disc and the inability of disc
cells to maintain or restore their extracellular matrix.-
Cause of this imbalance remains unknown.
-
Mediators of degradation
-
Proteolytic enzymes (cathepsin and lysozyme)
-
Inflammatory cytokine IL-1 decreases rate of proteoglycan production and increases rate of matrix breakdown.
-
-
-
Extrinsic factors: mediate degeneration of the disc by nutritional and/or vascular means (Box 23-1)
-
Mechanical environments
-
Overload hypothesis
-
Demanding mechanical environment produces
local trauma of the disc that will be slow to heal due to slow turnover
of the disc tissue. -
Accumulation of injury and microtrauma
progressively weakens the disc, making it more susceptible to further
injury, thus starting a vicious cycle.
-
-
Hypomobility hypothesisTable 23-1 Summary of Disc Mechanical Function
Structure Composition Function Outer anulus Dense concentric lamellae Resist tensile loads
Hydrostatic barrier that limits deformation
Reduce strains across vertebral bodiesInner anulus Less dense lamellae
Increased water content“Shock absorbers”: viscoelastic dissipation of force Nucleus pulposus High concentration of proteoglycans
Increased water contentResist axial compressive loads Endplate Hyaline cartilage Transfer of axial loads to vertebral body Table 23-2 Age-Related Changes in the DiscAge Group Changes Newborn Distinct hyaline cartilage endplates
Numerous perivascular and free nerve endings
Small amounts of collagen in nucleus
Small blood vessels present in outer lamellae
Proteoglycan in nucleus similar to endplateChildhood and adolescence Disc volume and diameter increase.
Blood vessels decrease in size and number.
Increase in cartilaginous content of anulus
Aggrecan becomes predominant proteoglycan.Adult Remaining peripheral vessels disappear.
Inner anulus expands.
Size of nucleus decreases.
Myxomatous degeneration of anulus; loss of collagen fiber organization
Fissures and cracks in lamellae
Concentration of viable cells declines.
Proteoglycan and water concentrations decrease.
Collagen and noncollagenous protein concentrations increase.
Decrease in structural integrityElderly Inner anulus and nucleus become fibrocartilage.
Few viable cells remain.
Decrease in height![]() Figure 23-8 Proposed pathway of cellular degeneration of the disc.P.457
Figure 23-8 Proposed pathway of cellular degeneration of the disc.P.457-
Hypomobility results in adaptive changes that may predispose to weakness and degeneration.
-
Resultant weakness and degenerative changes can cause pain, which further reduces motion and initiates another vicious cycle.
-
-
-
Genetics: plays a significant role in the variability of disc degeneration in the population samples studied to date (Box 23-2)
-
First reports of gene forms associated with intervertebral disc degeneration in humans were published in 1998.
-
Low magnetic resonance imaging (MRI)
signal intensity of thoracic and lumbar discs (disc desiccation)
associated with TaqItt-genotypes of the vitamin D-receptor gene
-
-
Several mechanisms have been suggested through which genetic factors could influence degenerative disc findings:
-
Size and shape of spinal structures
-
Intracellular processes that maintain disc function
-
-
Interactions of genetic and environmental factors are complex and continue to be an area of intense study.
-
-
Diminished material and structural properties
structure and composition of disc tissues, changes occur in the
material and structural properties of the components of the disc (Fig. 23-9). The degenerative changes in material properties are most profound in the nucleus and endplate.
-
Nucleus pulposus
-
Shear modulus of the nucleus increases eight-fold (becomes stiffer).
-
This decrease in energy dissipation
suggests that the nucleus pulposus undergoes a transition from
fluid-like to solid-like behavior. -
These alterations may be explained by the loss of water content and increase in tissue density.
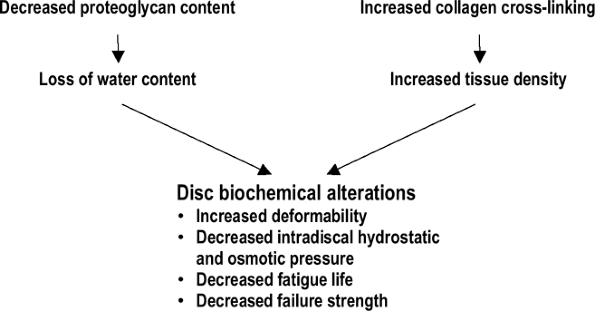 Figure 23-9 Biomechanical property changes of the degenerated disc.
Figure 23-9 Biomechanical property changes of the degenerated disc. -
This transition to more of a solid-like
state suggests a more anisotropic (orientation-dependent) stress state
with more non-uniform distribution of stresses.
P.458 -
-
Anulus fibrosus
-
Significant increase in compressive modulus
-
Decrease in radial permeability
-
Decrease in permeability results from loss of water content and obstruction of pores with debris.
-
Diffusion of nutrients, which relies on this permeability, is hindered.
-
-
Moderate increase in shear modulus
-
-
Endplate
-
Thinning, microfracture, or damage to the
endplate increases its hydraulic permeability, which allows rapid fluid
exudation with loading. -
This leads to a more non-uniform distribution of load as well as higher shear stresses that result in damage to the disc.
-
These compositional and structural
changes lead to non-uniform load transfers that result in high shear
stresses and material failures.
-
|
|
is poorly understood. Many factors, including structural changes in the
spine, soluble mediators that sensitize nerve endings, and nerve/vessel
ingrowth into the outer anulus, have all been hypothesized to be
possible causes of this chronic pain (Fig. 23-10).
-
Changes in the mechanical properties of
the disc lead to loss of spinal mobility and abnormal loading of the
facet joints, spinal ligaments, and surrounding muscles.-
The altered alignment and relationship may contribute to spinal pain.
-
-
The degenerated disc has been shown to produce cytokines and mediators that can sensitize surrounding nerve endings.
-
Tumor necrosis factor-alpha (TNF-α) has recently been suggested to play a role in discogenic pain.
![]() Figure 23-10
Figure 23-10
Pain fibers can penetrate more deeply into degenerated discs. These
fibers may be accompanied by vascular ingrowth. (From Wetzel FT.
Microinnervation: Pain generators. In Bono CM, Garfin SR, eds. Orthopaedic Surgery Essentials: Spine. Philadelphia: Lippincott-Raven, 2004:272–277.) -
TNF-α and other pro-inflammatory cytokines are a target of current research in pharmacologic intervention.
P.459 -
composition are observable with multiple imaging modalities. Although
plain radiographs are nearly always the first step, computed tomography
(CT) and MRI provide excellent detailed anatomic images of the spine.
Prior to their development, more invasive techniques such as
myelography, epidural venography, and epidurography were performed to
evaluate intradiscal pathology.
-
Early endplate changes preceding established degenerative disc diseases
-
Sclerosis, Schmorl’s nodes, calcifications
-
Observed on both radiographs and MRI
-
-
MRI is the advanced imaging modality of choice for intervertebral disc degeneration.
-
Characterizes many of the distinguishing features of the intervertebral disc
-
Does not distinguish between symptomatic and asymptomatic patients
-
Does not differentiate degeneration from age-related changes
-
Most sensitive MRI sign for disc degeneration: nucleus pulposus loses signal intensity on T2-weighted images
-
Loss of proteoglycans and dehydration occurring in degeneration
-
Seen as loss of signal intensity on T2-weighted images
-
-
-
Plain x-ray and MRI changes of degeneration
-
Loss of disc height
-
Osteophyte formation
-
Attributed to a compensation mechanism to distribute the increasing axial load and shear forces onto a larger bearing surface
-
These osteophytes have been differentiated into two types: traction and claw.
-
Traction osteophytes result from abnormal shear and are a sign of instability.
-
Claw-type osteophytes represent traction at the site of osseous attachment (Sharpey fibers) of the anulus fibrosus.
-
-
-
Radiographic correlates to morphological degree of degeneration
-
Plain film significant correlates: height loss, osteophytes, and intradiscal calcification (Table 23-3)
-
MRI correlative parameters: DEBIT (disc extension beyond the interspace), nucleus pulposus shape, anular tears, osteophytes, and endplate irregularity
-
-
New and potentially useful imaging
techniques for spine (continue to offer more opportunities to
investigate and diagnose back pain and intervertebral disc degeneration)-
Dynamic CT and MRI
-
Diffusion imaging
-
Magnetic resonance spectroscopy
-
-
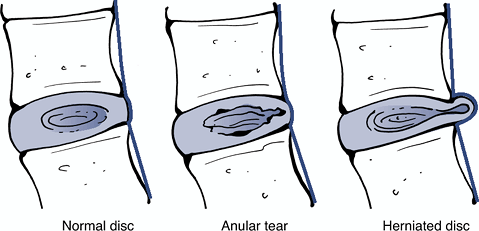
Definition: protrusion of tissue from the nucleus pulposus through a defect in the anulus fibrosus (Fig. 23-11)
-
Early clinical course
-
Initially fragmentation rarely perceived due to poor innervation of disc
-
Back pain typically experienced as outer anulus becomes involved with extension of the fissure and fragmentation
-
With the herniation of the discal components, pressure on the anulus is transferred to the nerve root.Table 23-3 Thompson Grading Scheme for Gross Morphology of the Human Lumbar Intervertebral Disc
Grade Nucleus Anulus Endplate Vertebral Body I Bulging gel Discrete fibrous lamellae Hyaline, uniformly thick Margins rounded II White fibrous tissue peripherally Mucinous material between lamellae Thickness irregular Margins pointed III Consolidated fibrous tissue Extensive mucinous infiltration; loss of anular-nuclear demarcation Focal defects in cartilage Early chondrophytes or osteophytes at margins IV Horizontal clefts parallel to endplate Focal disruptions Fibrocartilage extending from subchondral bone, irregularity and focal sclerosis in subchondral bone Osteophytes <2 mm V Clefts extend through nucleus and anulus — Diffuse sclerosis Osteophytes >2 mm 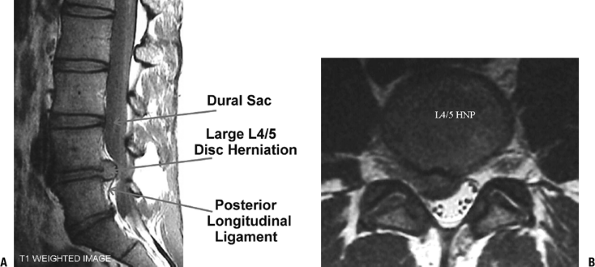 Figure 23-11 Lumbar microdiscectomy. MRI views of a large L4–5 disc herniation, sagittal (A) and axial (B). (From Koval KJ, Zuckerman, JD. Atlas of Orthopaedic Surgery: A Multimedia Reference. Philadelphia: Lippincott Williams & Wilkins, 2004.)
Figure 23-11 Lumbar microdiscectomy. MRI views of a large L4–5 disc herniation, sagittal (A) and axial (B). (From Koval KJ, Zuckerman, JD. Atlas of Orthopaedic Surgery: A Multimedia Reference. Philadelphia: Lippincott Williams & Wilkins, 2004.) -
Back pain is typically relieved at this point, yet the radiculopathy (sciatica) may increase in intensity.
P.460 -
-
Later clinical course and outcome
-
In >90% of patients with symptomatic disc herniations, the pain subsides within 3 months.
-
In many people (28% to 35%), disc
herniation occurs in the absence of symptoms. This relief of symptoms
has theoretically been attributed to a spontaneous resorptive process
that appears to be modulated by an inflammatory pathway.
-
-
Histopathology and healing process
-
Herniated disc is surrounded by granulation tissues with an inflammatory cell infiltrate and newly formed vessels.
-
Neovascularization is related by MRI to the resorption of the herniated disc.
-
Infiltrating macrophages also play a crucial role in this resorption.
-
-
Terminology from the North American Spine Society (NASS)
-
Herniation: localized displacement of disc material beyond the limits of the intervertebral disc space (Fig. 23-12).
The disc material may be nucleus, cartilage, fragmented apophyseal
bone, anular tissue, or any combination. The interspace is defined
cranial and caudad by the vertebral endplates and peripherally by the
outer edges of the vertebral ring apophyses (Fig. 23-13). -
Anular tear: localized radial,
concentric, or horizontal disruption of the anulus without associated
displacement of the disc material beyond the limits of the
intervertebral disc space (see Fig. 23-12) -
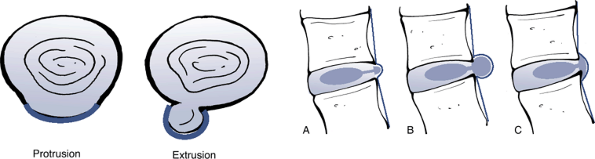
Extrusion: In at least one plane, any one
distance between the edges of the disc material beyond the disc space
is greater than the distance between the edges of the base measured in
the same plane; or when no continuity exists between the disc material
beyond the disc space and that within the disc space (Fig. 23-14). -
Protrusion: The greatest plane, in any
direction, between the edges of the disc material beyond the disc space
is less than the distance between the edges and the base (see Fig. 23-14).
-
-
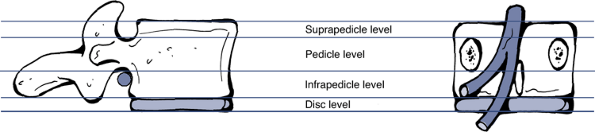
Location of the herniation: Wiltse proposed a system of anatomic zones and levels to characterize the herniation (Figs. 23-15 and 23-16).
![]() Figure 23-12
Figure 23-12
Sagittal anatomic sections showing the differentiating features of
anular tear and herniated disc. (After Milette PC, Fardon DF.
Nomenclature and classification of lumbar disc pathology. Spine 2001; 26: E93–E113.)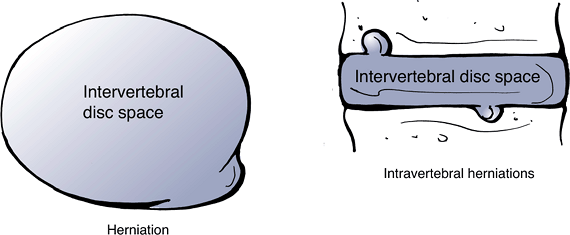 Figure 23-13
Figure 23-13
Herniation of disc material beyond the interspace can be in either the
axial or the caudad/cranial planes. (After Milette PC, Fardon DF.
Nomenclature and classification of lumbar disc pathology. Spine 2001;26: E93–E113.)![]() Figure 23-14
Figure 23-14
Differentiating characteristics of protrusion (A) and extrusion (B,C).
(After Milette PC, Fardon DF. Nomenclature and classification of lumbar
disc pathology. Spine 2001;26:E93–E113.)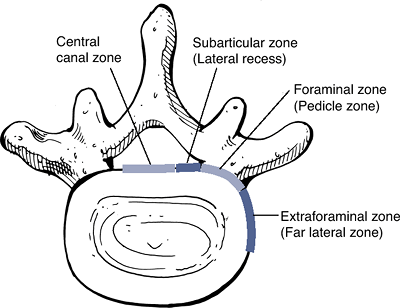 Figure 23-15
Figure 23-15
The anatomic “zones” identified on axial images. (After Milette PC,
Fardon DF. Nomenclature and classification of lumbar disc pathology. Spine 2001; 26:E93–E113.)![]() Figure 23-16
Figure 23-16
The anatomic “levels” identified on cranio–-caudad images. (After
Milette PC, Fardon DF. Nomenclature and classification of lumbar disc
pathology. Spine 2001;26:E93–E113.)
LM, Heini PF, Anderson SE, et al. Correlation of radiographic and MRI
parameters to morphological and biochemical assessment of
intervertebral disc degeneration. Eur Spine J 2005;14:27–35.
IAF, Iatridis JC. Mechanical conditions that accelerate intervertebral
disc degeneration: overload versus immobilization. Spine 2004;29:2724–2732.