RHEUMATOID ARTHRITIS OF THE CERVICAL SPINE
VIII – THE SPINE > Rheumatoid Disease > CHAPTER 154 – RHEUMATOID
ARTHRITIS OF THE CERVICAL SPINE
cervical spine is extremely common among patients with rheumatoid
arthritis. Estimates of frequency vary. Conlon et al. (16)
documented radiographic changes in the cervical spine for 85% (283 of
333) of patients with classic rheumatoid arthritis. While the majority
of such patients do not develop significant neurologic deficits,
identification of those at high risk of neurologic compromise remains a
difficult clinical problem.
rheumatoid involvement of the cervical spine are referred to as
atlantoaxial instability, cranial settling, and subaxial instability.
Clinically relevant radiographic measurements associated with the risk
of neurologic compromise have been described for these instability
patterns (4). In addition, advanced
radiographic techniques such as magnetic resonance imaging (MRI) allow
a more precise determination of spinal cord compression.
significant neurologic compromise, intractable pain, or both. As the
concept of impending neurologic compromise has been defined, the
selection of patients at risk for neurologic injury for surgical
stabilization has also improved. Given the uncertainty of recovery once
significant neurologic deficits are present, early stabilization of the
unstable rheumatoid spine appears to improve the outcome for these
patients. In addition, continued developments of surgical and
anesthetic techniques have facilitated their management.
(IgG and antibodies to IgG) deposits in the articular cartilage and
synovium of involved joints includes proliferation of fibrovascular
tissue, known as pannus. Examination of these tissues shows the
presence of chronic and acute-phase inflammatory cells, including
lymphocytes, plasma cells, and macrophages. The persistent inflammation
leads to cartilage loss and bony erosion, as well as ligamentous
laxity. In addition, the rheumatoid disease process per se leads to
diffuse osteopenia. Chronic steroid use may contribute to these
ligamentous and osseous changes as well.
rheumatoid arthritis because of the large number of articulations and
their significant mobility. The subaxial facet joints and
intervertebral discs as well as the ligaments and bursae of the
cervical spine are all potential locations of involvement (10,25).
The most common clinical involvement, however, includes the
atlanto-occipital, the atlantoaxial, the periodontoidal, and the
zygoapophyseal (facet) joints.
rheumatoid patients are anterior atlantoaxial instability, cranial
settling, and subaxial instability (Fig. 154.1) (16,43,54).
In addition to these patterns, other observed instability types have
included posterior instability of the atlas, subaxial dislocation, and
rotation and lateral subluxation of the atlas (5,26,42,45 and 46,50,57,60).
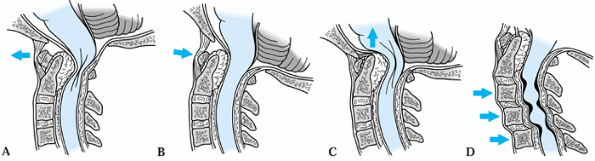 |
|
Figure 154.1. Patterns of cervical spine instability due to rheumatoid arthritis. A,B: Atlantoaxial instability. C: Cranial settling. D: Subaxial instability.
|
involvement is the development of neurologic compromise due to
compression of the spinal cord and nerve roots. This compression can
arise as a dynamic phenomenon due to the instability, or it can be
secondary to a mass effect caused by fixed vertebral subluxations or
pannus formation. In addition, deficits can arise that are attributable
to compromise of vascular supply at the level of either the anterior
and posterior spinal arteries or the vertebral arteries themselves (19,21,56).
radiculopathy, myelopathy, and cranial nerve compromise. The most
common radicular complaint is suboccipital headache due to irritation
of the second cervical nerve root by atlantoaxial degeneration or
instability (14,43).
Radiculopathy can also produce motor weakness due to disc collapse or
instability in the subaxial spine. The symptoms of myelopathy are
hyperreflexia and spasticity, with or without motor weakness. The
Ranawat classification of neurologic compromise has been widely used in
published reports of rheumatoid patients. Normal patients are
considered grade I, patients with paresthesias and hyperreflexia but
without motor weakness are grade II, and patients who demonstrate motor
weakness constitute grade III. Grade IIIA describes ambulatory
patients, and grade IIIB is used for nonambulatory patients (43).
may be asymmetric, and it may show greater involvement of the upper
extremities. Cruciate paralysis (of Bell) develops in some patients,
with a striking lack of weakness in the lower extremities but profound
involvement of the upper extremities due to medullary compression at
the pyramidal decussation of upper extremity motor fibers (3,65).
Cranial nerves, particularly the lower cranial nerves such as cranial
nerve IX (involving the gag reflex), can also be compromised,
especially in patients with cranial settling. Finally, respiratory
paralysis may also occur with upper cervical involvement, sometimes
with fatal results (16).
arthritis can be notoriously difficult because of the effects of
extremity contracture, deformity, pain, and inflammation, as well as
weakness from muscle wasting and mechanical
loss
of function. A high index of suspicion in patients complaining of neck
and occipital pain or new extremity weakness or loss of function is
therefore important. Descriptions of an electric shock sensation with
head and neck motion (Lhermitte’s sign) should also arouse suspicion.
radiographs, including an open-mouth odontoid view and lateral
flexion–extension views. These films may disclose osteopenia, erosion
of the atlantoaxial and subaxial facet joints, or erosion of the
odontoid process itself. While plain radiographs do not directly
demonstrate synovial pannus or spinal cord compression, much indirect
information may be gained, such as the presence of bony instability.
flexion–extension cervical spine radiographs. Historically, instability
was measured as the change in the anterior atlantodental interval
(AADI) (Fig. 154.2). Posterior displacement
greater than 3 mm of the dens relative to the anterior ring of the
atlas is considered abnormal. Displacement ranging from 6 to 10 mm has
been considered an indication for surgery, even in patients without
neurologic abnormalities (14,15,27,43,51).
 |
|
Figure 154.2.
Measures of atlantoaxial instability. The anterior atlantodental interval (AADI) measures mobility between the anterior ring of C-1 and the dens. The posterior atlantodental interval (PADI) also measures this mobility but also directly measures the space available to the spinal cord. The PADI has been shown to more accurately predict neurologic impairment than the AADI. |
cord compromise due to atlantoaxial instability is the posterior
atlantodental interval (PADI). Also referred to as the space available
to the cord, the PADI is measured from the posterior aspect of the dens
to the anterior edge of the posterior ring of the atlas, along the
transverse axis of the ring of the atlas (Fig. 154.2). Boden et al. (4)
demonstrated that a PADI of less than 14 mm correlated with a
significant risk of neurologic impairment, and that the PADI was a
better predictor of not only the development of neurologic compromise
but also the potential for neurologic recovery.
Chamberlain’s line runs from the posterior foramen magnum to the hard
palate. Wackenheim’s line is drawn tangent to the cranial surface of
the clivus. McGregor’s line runs from the lowest point of the occiput
to the hard palate. McRae’s line extends between the basion and the
posterior edge of the foramen magnum. For the projection of the dens
above
McGregor’s line, 4.5 mm is considered the upper limit of normal (12,20).
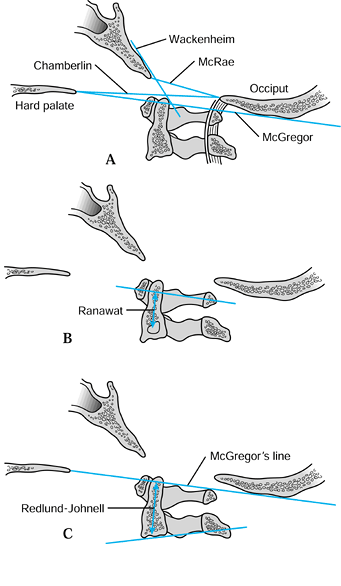 |
|
Figure 154.3. Measures of cranial settling. A:
Radiographic landmarks of the craniocervical junction. Chamberlain’s line extends from the posterior foramen magnum to the hard palate. Wackenheim’s line is a tangent to the cranial surface of the clivus. McGregor’s line extends from the lowest point of the occiput to the hard palate. McRae’s line extends between the basion and the posterior edge of the foramen magnum. B: Ranawat’s measure of cranial settling extends from the center of the pedicle of the second cervical vertebra to the transverse plane of the ring of the atlas. C: The Redlund-Johnell method measures the distance from the base of the axis to McGregor’s line. |
measurement from the center of the pedicle of the second cervical
vertebra to the transverse axis of the ring of the atlas (Fig. 154.3B).
They found normative values for this distance of 17 mm in men and 15 mm
in women. This measurement has the advantage that visualization of the
dens and skull base is not required.
Less than 34 mm in men or 29 mm in women indicates cranial settling.
The Sakaguchi-Kauppi method involves determination of the station of
the medial aspect of the superior facet of the axis relative to the
anterior ring of the atlas. These authors (29)
felt that their method was easier to apply than the Redlund-Johnell
method, and that it had the advantage of not relying on visualization
of the skull base or odontoid tip.
spine provides excellent detail of the bony structures. Erosion of the
dens and facet joints is much better demonstrated on CT images than on
plain radiographs (6). In addition, fractures of the dens may be diagnosed with this modality.
not only diagnostically, but also in some cases for surgical planning.
Posterior atlantoaxial arthrodesis using transarticular screws requires
sufficient width of the cervical two-vertebral isthmus to allow passage
of a 3.5 mm screw (35). In addition, sclerosis
and erosion of the posterior ring of the atlas may result in
insufficient bone stock for arthrodesis with conventional atlantoaxial
wiring techniques and thereby require atlantoaxial screw fixation or
extension of the arthrodesis to the occiput (14). There can be problems even with these techniques, however, when there is significant bone loss.
modality for evaluation of neurologic compression; use it to evaluate
any patient who has neurologic weakness or spasticity (7,30,31).
MRI provides enhanced definition of soft tissues and can demonstrate
spinal cord compression from pannus at the atlantodental articulation.
Recently, use of dynamic flexion–extension MRI views has been
recommended for preoperative planning in patients with neurologic
compromise (34). Patients for whom neurologic
compression is not relieved by maximal reduction of the atlantodental
articulation may be candidates for posterior decompression either by
removal of the posterior rim of the foramen magnum and laminectomy of
the atlas, or by anterior resection of the odontoid.
has been demonstrated with somatosensory evoked potentials and
cutaneous electric stimulation, both peripherally and in the trigeminal
nerve distribution (58). While such techniques
may confirm neurologic impairment, they are not widely used by
orthopaedic surgeons or neurosurgeons for diagnosis or treatment
planning, although they may have expanded roles in the future.
instability, some patients with rheumatoid arthritis develop severe
neurologic impairment with profound motor deficits, occasionally
leading to respiratory paralysis and death. It is also clear that once
neurologic deficits develop, there is no universally successful means
of regaining lost function. Unfortunately, our understanding of which
factors predict neurologic progression remains incomplete (12).
provided an early estimate of the incidence of cervical instability
among an unselected group of 333 rheumatoid patients. Plain radiographs
disclosed atlantoaxial subluxation in 84 (25%) of their patients, while
an additional 23 (7%) demonstrated subaxial subluxation. Although
cervical instability was statistically correlated with the severity of
peripheral disease, no correlation was found with duration of disease
or use of steroid medications. Although 23 patients (7%) demonstrated
symptoms of spasticity, the authors did not feel that these findings
correlated with the presence of cervical instability.
rheumatoid arthritis patients with significant atlantoaxial instability
but without neurologic compromise at the time of initial radiographs.
They reevaluated 84 surviving patients an average of 7.8 years after
the initial examination. Four patients (3%) had developed spinal cord
compromise, while an additional six (5%) described symptoms of
transient weakness. Of the 84 (74%) surviving patients, 62 had been
maintained on chronic oral steroid medication, which appeared to
correlate with radiographic progression of instability. No effect of
cervical instability on long-term survival could be demonstrated.
followed 100 patients prospectively with annual flexion–extension
radiographs. Over an average of 7 years’ follow-up, they documented
that 12 patients (12%) developed atlantoaxial instability, 8% developed
subaxial instability, and 3% developed cranial settling. All the
patients who developed instability demonstrated onset of subluxations
within 2 years of diagnosis with rheumatoid arthritis. By an average of
9 years and 5 months’ follow-up, one patient had developed myelopathy
and two had undergone posterior cervical fusion for severe occipital
headache (3%). These authors also demonstrated a significant
correlation between the presence
of severe peripheral erosive disease and cervical spine involvement.
the progression of symptoms in 16 patients with 8 mm or greater
atlantodental instability or cranial settling. They compared disease
progression in these patients with a group of 18 surgically treated
patients with a comparable degree of radiographic instability but more
significant neurologic symptoms. Although this was not a randomized
study, they found that none of the operatively treated group had
worsening of their neurologic status, and 8 of 14 (57%) who had had
preoperative neurologic deficits showed improvement. Postoperative
complications were relatively minor. In the nonoperatively treated
group, however, 7 of 14 surviving patients (50%) suffered neurologic
worsening. A further report on this patient group documented
progression of cranial settling in 12 untreated patients, three of whom
developed neurologic progression (52).
rheumatoid arthritis patients with initial complaints of cervical pain
over a 5-year period (40). They noted
neurologic progression in 27 of 85 surviving patients (36%) and
radiographic progression in 60 (80%). Seven patients had undergone
surgical intervention by the end of the study secondary to severe
neurologic involvement. Only two patients underwent spontaneous fusion
of the atlantoaxial articulation, one of whom subsequently developed
subaxial subluxations (Fig. 154.4). They also
found that patients without radiographic changes at the time of their
initial complaints did not develop significant instability over the 5
years of the study.
 |
|
Figure 154.4. An adverse natural history. A,B:
Flexion–extension lateral radiographs obtained in 1991 demonstrate significant atlantoaxial instability, although the patient was neurologically normal. Significant erosion of the dens was already present, allowing posterior displacement of the ring of C-1 in extension. C,D: Six years later, a fixed posterior subluxation of C-1 has developed. Subaxial subluxation is also present. The patient at this time was quadriparetic and unable to ambulate (Ranawat IIIB). E: A sagittal view from an MRI scan demonstrates cord compression at both the atlantoaxial and subaxial levels. This patient would likely have benefited from an atlantoaxial arthrodesis at an earlier stage. |
patients followed for an average of 7 years, 42 of whom (58%) developed
neurologic compromise. These authors demonstrated the importance of the
PADI as a predictor for neurologic injury (Fig. 154.2).
They noted that a reduction of the PADI below 14 mm, or a reduction of
the spinal canal diameter below 14 mm in the subaxial cervical spine,
was correlated with an increased prevalence of nonrecoverable
neurologic deficit. They also demonstrated an increased risk of
neurologic deficit in patients with atlantoaxial subluxation combined
with cranial projection of the odontoid of 5 mm or greater above
McGregor’s line.
improve. Many patients are now maintained on methotrexate or other
nonsteroid medical regimens, with steroid use limited to short-duration
bursts for flares of the rheumatoid disease. The effect of this shift
in treatment patterns on the progression of cervical instability is not
known.
other operative procedures requiring general anesthetic should undergo
cervical radiographic evaluation with dynamic flexion–extension lateral
views. Patients with neck pain but without significant instability can
be treated with pain medication and a cervical orthosis. While
orthotics may provide symptomatic relief, they neither slow the disease
process nor provide significant additional stability, and these
patients should be followed for possible progression of their disease (1).
in surgical patients with rheumatoid arthritis. Patients undergoing
multiple-level anterior cervical spine procedures and those with severe
neurologic compromise should be considered for elective tracheostomy (27).
If a tracheostomy is not likely to be necessary, patients may need to
remain intubated postoperatively for an additional period of time. All
other rheumatoid patients should be considered for fiberoptic
intubation (14). This a good practice for all
neurologically vulnerable patients, and a reduction in postoperative
airway complications in rheumatoid patients undergoing fiberoptic
intubation has been demonstrated (59).
patients with cranial settling or subluxations that do not reduce on
voluntary flexion–extension lateral radiographs. Traction for 24–48
hours has proven useful, with most reductions occurring within this
time (38). Extended periods of traction
probably do not improve reduction and should generally be avoided
because of the preexisting physical weakness of many patients with
rheumatoid arthritis and the rapid physical deterioration that occurs
with extended bed rest. Halo-wheelchair traction provides traction
while allowing the patient to be upright.
individual patient’s needs. Historically, several means of
immobilization have been used ranging from skull traction or a halo
cast to a cervicothoracic or hard cervical orthosis (14,16,27,43,66).
Halo-bracing is generally well tolerated by rheumatoid patients,
perhaps because of their lower physical demands. The advantages of
reducing the risks of hardware failure and nonunion in this patient
population probably outweighs the easier mobility afforded by less
aggressive bracing.
surgery before deciding on a specific surgical plan; pay attention to
the patient’s activity level, expectations, and overall health status.
Preoperative evaluation should include pulmonary, cardiac, and renal
testing. Patients with longstanding, severe neurologic injury and
patients with limited pulmonary or cardiac reserve should be considered
for nonoperative management.
neurologic compromise demonstrated by spasticity or motor weakness
(Ranawat II or III), impending neurologic compromise as demonstrated by
spinal cord compression on MRI or a PADI of 14 mm or less on plain
films, and intractable occipital headache with demonstrated
atlantoaxial degeneration or instability. If preoperative reduction in
cranial traction is insufficient to allow spinal cord decompression,
consider a laminectomy of the atlas, and enlargement of the foramen
magnum as well, which usually requires extension of the arthrodesis to
the occiput.
arthrodesis have been described. Historically, posterior wiring
techniques have been very successful in other patient populations but
have had significant nonunion rates in patients with rheumatoid
arthritis. In addition, atlantoaxial wiring techniques require
availability of the posterior ring of the atlas. Recently, the
technique of transarticular screw fixation has improved fusion rates
and can be performed with laminectomy of the posterior ring of the
atlas. Risks of neurologic and vascular injury with this technique are
still being evaluated, however, and it should not be used when
reduction of the atlantoaxial facet joints cannot be achieved. A CT
scan with sagittal reconstructions to evaluate the position of the
vertebral arteries is a prerequisite for this technique.
They had less success in patients with rheumatoid arthritis, however,
describing one patient who developed nonunion and a second who suffered
an intraoperative fracture and required extension of the arthrodesis to
the occiput. Clark et al. (14) modified this technique by using a single, larger piece of corticocancellous bone graft (Fig. 154.5C).
They reported a bony fusion rate of 75% in a series of 20 patients with
rheumatoid arthritis, with an additional two patients achieving stable
fibrous union (Fig. 154.6).
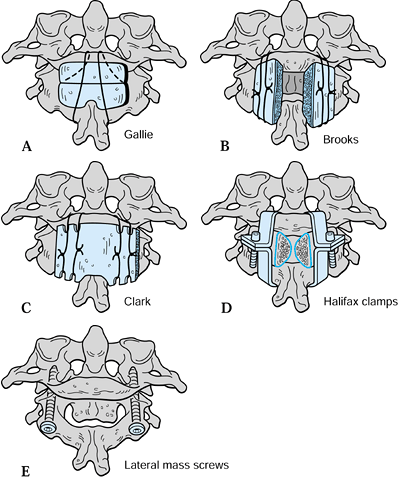 |
|
Figure 154.5. Methods of C-1/C-2 fixation. A: Gallie wiring. B: Brooks wedge-compression wiring. C: Clark wiring. D: Halifax clamps. E: Posterior transarticular screws.
|
 |
|
Figure 154.6. This female patient had longstanding rheumatoid arthritis with new-onset long-tract signs without motor weakness (Ranawat II). A,B: Flexion–extension lateral radiographs demonstrate significant atlantoaxial instability with full reduction in extension. C: MRI in extension demonstrates resolution of neurologic compression with reduction of the atlantoaxial articulation. D: Following posterior wiring and fusion, solid arthrodesis occurred with resolution of myelopathic symptoms.
|
from the atlas to the subaxial spine in a series of five patients with
either prior nonunions (two patients) or combined atlantoaxial and
subaxial subluxations (three patients). Two of these patients
progressed to nonunion, and a third patient developed a wound infection
and fistula. Because of this higher incidence of wound problems, as
well as reports of bone lysis, PMMA is no longer recommended as a
supplement to cervical fixation (37).
While the overall fusion rate in this series was 80% (20 of 25), the
rate for rheumatoid arthritis patients was only 73% (11 of 15). This
technique thus seems to offer little improvement over wiring techniques
with respect to fusion rate, although it may be neurologically safer
than sublaminar wires for patients with severe stenosis.
described posterior transarticular screw placement for atlantoaxial
stabilization and arthrodesis, this technique has been given increasing
attention (Fig. 154.5E). Grob et al. (24)
described their experience with transarticular 3.5 mm screws
supplemented with midline wiring in a series of 161 patients, including
51 with rheumatoid arthritis. They achieved a 99.4% fusion rate at an
average of 24 months. Although no symptomatic vertebral artery injuries
were reported, there were five postoperative deaths, three in patients
undergoing simultaneous transoral odontoid resection.
structures due to screw malposition continues to be evaluated. Madawi
et al. (35) reported an 87% fusion rate in 61
patients (37 rheumatoid patients) using transarticular screw fixation.
They reported that 14% of screws were malpositioned with an 8% (5 of
61) rate of vertebral artery injury, although only one patient was
symptomatic. These authors also described anatomic measurements on 25
cadaverous C-2 vertebrae, demonstrating an insufficient diameter of the
pars interarticularis to accommodate a 3.5 mm screw in 20% of
individuals. Recently published survey data from 847 neurosurgeons
regarding 1,318 patients treated with transarticular screws revealed a
rate of vertebral artery injury of 4.1%. Most arterial injuries were
asymptomatic, however, with only 0.2% of all patients suffering a
neurologic deficit (64).
the risk of vertebral artery injury in rheumatoid patients has not been
fully evaluated. This risk is probably somewhat higher than in the
nonrheumatoid population because of the tortuous anatomy of the
vertebral artery that can develop with rheumatoid arthritis, as well as
the difficulty of reducing the atlantoaxial facets in some patients,
complicating appropriate screw trajectory.
higher rate of arthrodesis, further documentation of outcome in
rheumatoid patients is needed. Despite a lower mechanical rigidity
compared with transarticular screw fixation, wiring techniques are
still appropriate in many patients because they offer a reduced risk of
neurologic and vascular complications, particularly when reduction of
the atlantoaxial articulation cannot be obtained, or when the isthmus
is smaller than 3.5 mm in diameter (53).
cervical arthrodesis include cranial settling with current or impending
neurologic compromise, inability to obtain fusion to the posterior ring
of the atlas due either to insufficient bone stock or to the need for a
laminectomy, nonunion from a prior atlantoaxial arthrodesis, and severe
involvement in patients with combined atlantoaxial and subaxial
instability. While inclusion of the occiput in the arthrodesis further
reduces neck motion over an isolated atlantoaxial fusion, incorporating
the occiput affords strong fixation, allowing a variety of constructs
for stabilization.
historically provided acceptable results in a large number of patients.
De Groote et al. (18) described results in 14 rheumatoid arthritis patients using an H-graft and a wiring technique based on the method of Robinson and Southwick (47).
They obtained fusion in 11 patients, and none of the three patients
with nonunion required revision surgery during the follow-up period.
using a spinous process wire at C-2, a looped sublaminar wire at C-1,
and a wire through the inion along with structural corticocancellous
bone grafting posteriorly. In a series of 13 patients, eight of whom
had rheumatoid arthritis, they achieved a 100% fusion rate with all
patients with preoperative neurologic deficits showing improvement.
Clark et al. (11,14) described a six-wire technique using paired lateral sublaminar wires at the atlas and axis (Fig. 154.7A). This method can still be used when a laminectomy of the atlas has been performed.
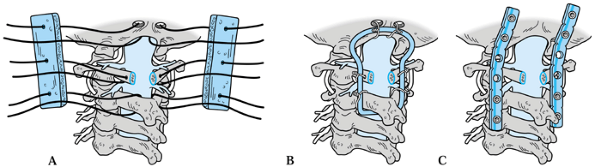 |
|
Figure 154.7. Methods of occipitocervical stabilization. A: Clark wiring. B: Ransford loop. C: Occipitocervical plating.
|
rheumatoid arthritis. They had an 85% fusion rate (33 of 37 patients).
They noted that when reduction of cranial settling was achieved and
maintained, patients had a significantly better prognosis for
neurologic recovery than when reduction was not possible (93% versus
40%). Only two patients underwent a late anterior odontoid resection
due to persistent compression and neurologic deficits; both eventually
recovered normal neurologic function.
occipitocervical fixation with a contoured, threaded Steinmann pin and
sublaminar wiring along with laminectomy of the atlas and foramen
magnum enlargement in a series of three patients (Fig. 154.7B).
Although none of the patients had rheumatoid arthritis, the authors
recognized the potential application to this population. Itoh et al. (28)
described 13 rheumatoid patients treated with this technique, fusing an
average of 5.9 cervical levels. Ten of these patients had cranial
settling, and 12 had subaxial involvement. Of 8 patients with moderate
or severe myelopathy, 7 (88%) had significant postoperative neurologic
improvement. Twelve of 13 patients (92%) went on to solid arthrodesis.
All patients had relief of occipital pain.
results with this technique in 39 patients, 12 of whom had rheumatoid
arthritis. Five of the 12 rheumatoid patients (42%) had cranial
settling, while 4 (33%) had prior nonunions. Four patients (33%)
underwent foramen magnum enlargement and laminectomy of the atlas,
while 5 (42%) underwent transoral resection of the odontoid. Ten of
these patients (83%) went on to solid arthrodesis, with 2 developing
stable fibrous union. None of these patients suffered hardware failure.
All 10 patients with preoperative myelopathy showed improvement,
although 9 (90%) demonstrated persistent deficits to varying degrees.
Like transarticular screw fixation for atlantoaxial arthrodesis, this
technique was developed in Europe but is increasingly used in the
United States as well. The advantages claimed for occipitocervical
plating include avoiding entry into the spinal canal and reducing the
number of caudal segments required to obtain rigid
fixation. An early report by Grob et al. (23) described this technique in 14 patients, seven of whom had rheumatoid arthritis. Using a Y-shaped
plate with a single arm for cranial fixation and including
transarticular atlantoaxial screws as part of their construct, they
reported fusion in all patients.
preliminary results in 14 patients using bilateral, contoured steel
pelvic 3.5 mm reconstruction plates. They used a pedicle screw at the
C-2 vertebra in place of transarticular screws. Five of these patients
had rheumatoid arthritis, and the arthrodesis extended an average of
4.6 cervical levels. Fusion was reportedly obtained in all patients.
While these early results are promising, long-term follow-up of these
patients to evaluate for instability caudal to the arthrodesis is
needed.
these techniques require training and experience prior to routine use.
The specific risks of neurologic or vertebral artery injury during
lateral mass or atlas pedicle screw placement have not been documented
in rheumatoid arthritis patients, where marked distortion of the
anatomy can occur. A CT scan to determine the course of the vertebral
arteries is advised. While these techniques offer the potential to
improve fusion rates and reduce postoperative immobilization, the
long-term effects of these constructs on adjacent motion segments is
also unknown.
indicated for patients with subaxial instability or fixed subluxation
with impending or actual neurologic compromise. As with atlantoaxial
instability, a measurement of the space available to the cord on a
lateral radiograph of 14 mm or less indicates that the spinal cord is
at risk of compromise. Patients with subaxial instability who also have
atlantoaxial instability or cranial settling often require treatment of
these combined instability patterns by a single operation (4).
subaxial rheumatoid spine problems than of upper cervical spine
involvement. Ranawat et al. (43) discussed
posterior arthrodesis in six patients with subaxial subluxations. Three
of these patients underwent arthrodesis to the occiput due either to
coexisting atlantoaxial instability or cranial settling. All patients
had significant pain relief and three (50%) had improvement of
myelopathic symptoms.
treated with anterior surgery; no patient achieved neurologic
improvement, because of graft collapse and dislodgement. These authors
felt that anterior surgery was contraindicated in patients with
rheumatoid arthritis because of the mechanically insufficient,
osteopenic bone of the vertebral bodies (43).
In addition, the anterior longitudinal ligament may be one of the last
remaining stabilizers in rheumatoid patients, and this ligament is
necessarily disrupted during an anterior procedure.
results in seven patients with isolated subaxial involvement treated by
posterior fusion without wiring. Two patients underwent laminectomy due
to severe neurologic compromise, but neither recovered significant
function. Patients were managed postoperatively either in cranial
traction or a halo. All six surviving patients went on to solid
arthrodesis, with satisfactory results reported in 4 of 7 patients
(57%).
seven patients with subaxial instability, four of whom had had
preoperative myelopathy. Five patients treated with posterior subaxial
bone grafting and wiring went on to solid fusion, with all
neurologically compromised patients experiencing significant recovery.
No laminectomies were performed in this series. Two patients treated
with anterior procedures died postoperatively of pulmonary
complications. These authors argued against the need for a laminectomy
to obtain neurologic recovery, and they argued for posterior rather
than anterior procedures.
rheumatoid patients, seven of whom had posterior subaxial arthrodesis
for subaxial instability. They reported no subaxial nonunions with
spinous process wiring and bone grafting. Four patients (67%) had
clinical improvement of pain complaints, and no patient suffered
neurologic worsening. One patient who had undergone an anterior
corpectomy and attempted arthrodesis had developed graft subluxation,
which necessitated the posterior procedure.
results in 16 patients with subaxial instability treated with a
posterior procedure. Ten patients had myelopathy and seven had severe
neck and shoulder pain. All patients underwent posterior wiring and
arthrodesis, and patients with myelopathy also underwent laminectomy.
Eight patients were treated in postoperative skull traction. All
achieved solid fusion, and 90% (9 of 10) with myelopathy recovered. Two
perioperative deaths occurred. Three patients developed adjacent
segment instability during an average follow-up period of 4.4 years.
subaxial anterior decompression procedures in patients with rheumatoid
arthritis (14,27,43).
The reasons for failure seem to be the tendency of the osteoporotic
vertebral bodies to collapse around the bone graft, with resulting
nonunion, kyphosis, and graft extrusion. In most cases, rheumatoid
patients with subaxial instability and osteopenia or combined subaxial
and upper cervical involvement should be treated with posterior surgery.
anterior spinal cord compression following posterior fusion may be
candidates for anterior decompression and structural bone grafting. In
addition, there appears to be a subclass of rheumatoid patients with
less severe involvement that
can
be effectively treated with anterior decompression and arthrodesis
procedures. These patients may have fewer peripheral joint deformities,
less corticosteroid exposure, greater bone density, and a shorter
duration of rheumatoid disease. The radiographic appearance of these
patients is similar to that of patients with cervical spondylosis,
without significant subaxial or atlantoaxial instability (Fig. 154.8).
 |
|
Figure 154.8. Subaxial cervical degeneration treated by anterior corpectomy and fusion. A:
Lateral cervical spine radiograph demonstrates subaxial spondylosis in this 66-year-old man. He had a 20-year history of seropositive rheumatoid arthritis but displayed limited peripheral joint deformity. He was maintained on a combination of methotrexate and a daily dose of 5 mm prednisone. Despite the long history of rheumatoid arthritis, his clinical appearance is more consistent with cervical spondylosis without significant instability. B: Postmyelogram CT image through the C-5/C-6 disc level demonstrates significant spinal cord compression. Spasticity without motor weakness was present. The patient had undergone a laminoplasty with subsequent reclosure of the laminae. C: Sagittal MRI demonstrates spinal cord compression at the C-3/C-4, C-4/C-5, C-5/C-6, and C-6/C-7 discs. D: This patient underwent anterior corpectomy of C-4, C-5, and C-6 with autologous fibular strut grafting with successful fusion and resolution of his myelopathic symptoms. |
process is sometimes required because of severe cranial settling with
persistent neurologic compression despite maximal reduction in skeletal
traction. For many patients, decompression can be accomplished at the
time of occipitocervical arthrodesis via traction reduction with
foramen magnum enlargement and laminectomy of the atlas. For patients
who fail to improve despite this treatment or whose compression is so
severe that posterior decompression is likely to be inadequate,
anterior resection of the odontoid is indicated.
performed simultaneous odontoid resection and posterior
occipitocervical arthrodesis in 14 patients with rheumatoid arthritis,
all of whom had myelopathy with significant weakness. One patient
suffered a vertebral artery injury requiring abortion of the procedure.
No patient developed a wound infection. These authors argue that this
procedure results in faster neurologic recovery and avoids the need to
obtain intraoperatively and hold postoperatively an anatomic reduction.
Postoperative MRI imaging has documented reduction in the periodontoid
pannus following solid arthrodesis. In addition, significant numbers of
patients have demonstrated good
neurologic recovery with solid posterior arthrodesis in a reduced position (14,37).
Be aware of the fact that the physiologic stress and difficulties of
airway management during combined anterior/posterior cervical spine
surgery in this population are considerable. Finally, patients who do
not fully recover from neurologic deficits following arthrodesis may
obtain further neurologic recovery after a delayed anterior odontoid
resection (37).
-
After fiberoptic intubation and placement
of spinal cord monitoring leads, position the patient prone using tongs
or halo traction on a head rest to control the head position. Position
the head in sufficient extension to reduce the atlantoaxial
articulation, and verify the reduction fluoroscopically prior to
preparing and draping. Recheck neurologic status after positioning,
either via spinal cord monitoring or a brief wake-up test.
Alternatively, position patients while awake to allow continuous
neurologic monitoring prior to the induction of general anesthesia. -
Following a midline posterior approach,
expose the ring of the atlas; strip only 1.5 cm laterally on either
side of the midline to avoid injuring the vertebral arteries. -
Expose the C-2 and C-3 vertebrae to the lateral edge of the C-2 and C-3 facets, avoiding injury to the joint capsules.
-
Gently strip the attachments of
ligamentum flavum from the cranial and caudal edges of the laminae of
both the atlas and the axis vertebrae with a 4-0 curved curet. Pass a
threaded 1.5-cm-diameter French-eye needle, blunt end first,
sequentially under both laminae, retrieving the leading end with a
small needle driver. -
Carefully follow the curvature of the
needle during advancement under the laminae to avoid injuring the dura.
Repeat this maneuver until a total of four sutures (usually #2 Ticron)
are under the laminae of both the atlas and the axis. -
Tie a free end of one of the sutures to
the looped end of a 24-gauge wire that has been doubled on itself and
twisted to form a single strand. Gently pull this wire into position
under the lamina and detach the suture. -
Position the wire laterally and repeat
this maneuver with the remaining sutures to produce two pairs of
sublaminar wires on either side. -
Once the paired sublaminar wires are in
place, obtain a thick corticocancellous bone graft from the posterior
iliac crest. It should be a plate of cortical bone with the full
thickness of cancellous bone remaining underneath, measuring at least 2
by 4 cm. -
Shape the graft on its cancellous
surface, removing bone to form a wedge shape, thickest at the center
and diminishing toward the cranial and caudal edges. -
Notch the caudal edge of the graft centrally with a rongeur to accommodate the spinous process of the atlas.
-
Lightly decorticate the posterior elements of C-1 and C-2 with a burr.
-
Once the cancellous side of the graft
lies flush against the laminae of the atlas and axis, tighten one wire
by twisting with steady posterior tension. Tighten it until it is flush
with the cortical surface of the graft throughout its length and the
knot just begins to double on itself. -
Alternate sides until all four wires are
tight, cutting the ends and tamping them down to the cortical surface
of the bone graft. -
Following closure and dressing of the
wound, place the patient in a halo brace in neutral flexion–extension
to be worn for 12 weeks (Fig. 154.5C and Fig. 154.6).
-
Do not dissect farther than 1.5 cm lateral to midline at the ring of C-1 to avoid injury to the vertebral arteries.
-
Thoroughly free the cranial and caudal
attachments of the ligamentum flavum before attempting to pass the free
needle. A 4-0 curved curet should pass readily along the cranial and
caudal surfaces of the laminae. Do not, however, attempt to pass the
curet or any other instrument underneath the laminae. -
Once sutures or wires are in place, clamp free ends with a small hemostat to prevent entanglement.
-
Avoid breaking the graft during removal.
Thoroughly cut all four sides of the graft with an osteotome,
visualizing the end of the osteotome all the way to the end of the cut.
Do not lever the graft out until the deep surface of cancellous bone
has been completely separated from the deep cortex and the graft moves
freely.
-
Place the patient in three-point tongs
prior to positioning. Radiolucent tongs with a radiolucent operating
table improve fluoroscopic visualization of the atlantoaxial facet
joints. Position the patient prone, with the upper cervical spine in
slight flexion and the lower cervical spine in extension. This position
allows better access to the starting points and better trajectory for
screw placement. Check fluoroscopically that the atlantoaxial
articulation is reduced and that both facet joints are visible on the
anteroposterior (AP) view. Reassess neurologic function after
positioning, either by a brief wake-up test or by verification of
maintenance of baseline somatosensory evoked potentials. Position
patients with marked instability while they are awake to allow
continuous neurologic monitoring prior to inducing general anesthesia. -
Use a midline posterior cervical
approach. Take care not to expose the occiput, as the fusion mass can
unexpectedly extend to areas of exposed bone. -
Achieve the exposure described for the
atlantoaxial wiring and extend it cranially from the C-2/C-3 facets
along the isthmus of C-2 to expose the C-1/C-2 facet joint. Be careful
to remain subperiosteal and to perform the dissection bluntly to avoid
injury to the C-2 nerve roots exiting posterior to the C-1/C-2 facets.
Place a Penfield 4 elevator gently along the medial wall of the C-2
pedicle and into the C-1/C-2 facet joint to verify orientation. -
Obtaining a sufficiently steep trajectory
for screw placement can be very difficult. Start the screw on the
medial side of the inferior facet of the axis, aiming for the exposed
isthmus cranially. -
Under fluoroscopic or stereotactic
guidance, aim for the middle of the C-1/C-2 facet joint on the AP view,
and for the anterior ring of the atlas on the lateral view. -
Use a cannulated drill system to allow
repositioning of the guide wire until you are satisfied with the
orientation and location. -
The thoracic cage can obstruct hand
position and block the appropriate trajectory. If it does, a
percutaneous incision distally on the back can improve orientation. Use
a flexible, cannulated 2.5 mm drill and articulated screwdriver to
improve the inclination. -
Place both guide wires prior to drilling and screw placement, to stabilize the articulation during passage of the first screw.
-
Tap past the facet joint to improve ease
of screw insertion. After cannulated drilling and tapping, place either
a solid 3.5 mm or cannulated 4.0 mm screw. Use fully threaded
narrow-pitch screws for the best purchase. Screw length should come
just to the inferior edge of the anterior C-1 ring on the lateral view. -
Once screws have been placed, augment the
construct with a corticocancellous bone graft and wiring following the
previous guidelines (Fig. 154.5E, Fig. 154.9).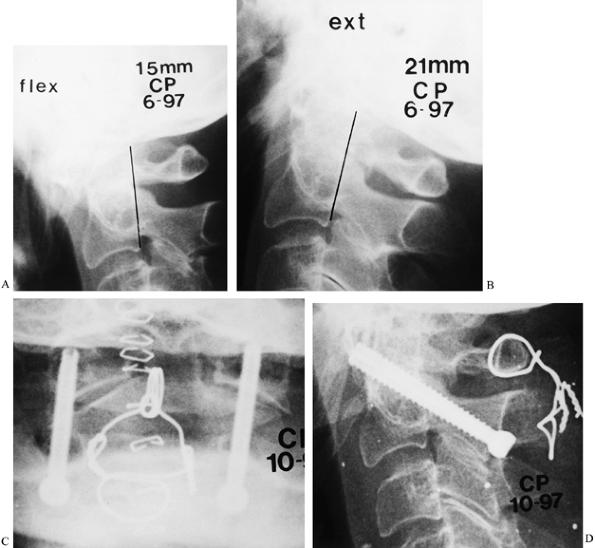 Figure 154.9. This 66-year-old woman had hyperreflexia in upper and lower extremities without weakness or pathologic reflexes. A,B:
Figure 154.9. This 66-year-old woman had hyperreflexia in upper and lower extremities without weakness or pathologic reflexes. A,B:
Flexion–extension lateral radiographs demonstrate reduction of the
posterior atlantodental interval to 15 mm in flexion. Full reduction
occurs with extension. C,D: Because of the
limited space available to the spinal cord, this patient underwent
posterior atlantoaxial arthrodesis with transarticular screw fixation
and supplementary wiring. She obtained a solid fusion with no
neurologic deterioration.See Hints and Tricks on the next page.
-
Prepare and position the patient as in
previous descriptions. Check atlantoaxial reduction radiographically
and verify neurologic status once positioning is complete. Prepare and
drape well the back of the head, approximately 5 cm cranial to the
inion. Use a posterior
P.4004
cervical
approach, extending the cranial exposure approximately 1 cm cranial to
the inion. Exposure of the posterior ring of the atlas should extend no
more than 1.5 cm lateral on either side.
-
Do not begin the procedure until fully
satisfied with the head position, visualization of necessary anatomy on
both AP and lateral fluoroscopic views, and reduction of the
atlantoaxial articulation. -
Verify on preoperative CT sagittal
reconstructions that the C-2 isthmus is sufficiently large to
accommodate at least a 3.5 mm screw. -
Extend preparation and draping distal to
the scapulae to allow percutaneous incisions for drilling and screw
placement if needed. -
A looped sublaminar wire at C-1 can
improve reduction and allow stabilization of the C-1 ring during
passage of the guide wires and screws. An alternative procedure such as
C-1 laminectomy and occipitocervical arthrodesis should be performed in
patients whose C-1/C-2 articulation cannot be anatomically reduced. -
Accurate assessment of screw orientation
in the coronal plane is critical. Lateral screw position can produce
injury to the vertebral artery, while medial positioning threatens
neurologic structures.
-
If a laminectomy of C-1 and foramen magnum enlargement are necessary, do these before preparing for wiring.
-
Free the ring of C-1 of ligamentous
attachments cranially and caudally with a sharp 4-0 angled curet. If
the ring is too thick to remove with a 1 mm Kerrison rongeur, it may be
thinned with a burr. -
Start the resection at the lateral limits
of the exposure of the laminae first, leaving a floating central
fragment to be resected last. This technique reduces the potential
pressure against the cord during the laminectomy. -
Enlarge the foramen magnum by thinning
the occiput with a burr in a semicircle measuring approximately 5–7 mm.
Then resect the remaining inner table piecemeal with a 2 mm Kerrison
rongeur to remove the posterior lip of the foramen magnum; always do
this to allow safe passage of occipital wires. -
Create two occipital burr holes with a 4
mm carbide burr approximately 1 cm lateral to the inion and
approximately 7 mm cranial to the foramen magnum. -
Complete the holes through the inner
table with a diamond burr. Elevate the dura off the inner table toward
the burr holes and from the foramen magnum with a 4-0 curved curet. -
Pass a looped, double-twisted 24-gauge
wire through the holes on both sides using the suture-passing technique
previously described. -
If a laminectomy of C-1 has been
performed, drill a small hole through the remnant of the lamina on
either side, if there is sufficient remaining bone, and pass a single
24-gauge wire through this hole. Otherwise, pass bilateral sublaminar
wires at both C-1 and C-2 using a technique similar to that described
for atlantoaxial wiring. -
Alternatively, if neurologic compression
is present, pass a wire through the spinous process of C-2 by drilling
transversely approximately a third of the length up the spinous process
from the laminae. Use a 2 mm burr to perforate the cortex on either
side of the spinous process and connect those holes with a towel clip.
Then pass a 20-gauge wire through the hole, loop it under the spinous
process, and pass it a second time. Use a similar method if the fusion
is to be extended caudally; we do not use sublaminar wires caudal to
C-2. -
As in atlantoaxial arthrodesis, obtain a
thick corticocancellous bone graft from the posterior iliac crest. For
occipitocervical arthrodesis, harvest a graft measuring approximately 3
by 5 cm. -
Divide the graft lengthwise and place three evenly spaced drill holes in both grafts.
-
Lightly decorticate the occiput, C-1 ring (if still present), and C-2 laminae using a carbide burr.
-
Thread the more lateral arm of the wire
at each level through the corresponding holes, and maneuver the graft
down the wires until it is in apposition to the decorticated bone.
Bring the second arm of each wire medially around the graft and tighten
the wires sequentially as described previously. -
If fixation is secure and bone quality is
good, use a skull–occiput–mandibular immobilization (SOMI) or Minerva
brace postoperatively. If fixation is compromised because of osteopenic
bone, maintain the patient in a halo vest for 6–12 weeks after surgery (Fig. 154.7A, Fig. 154.10).![]() Figure 154.10. This patient had hyperreflexia and bilateral Babinski and Hoffman’s signs without motor weakness (Ranawat II). A,B:
Figure 154.10. This patient had hyperreflexia and bilateral Babinski and Hoffman’s signs without motor weakness (Ranawat II). A,B:
Flexion–extension lateral radiographs demonstrate atlantoaxial
subluxation with incomplete reduction in extension. Posterior
atlantodental interval (PADI) is 11 mm. Mild cranial settling is also
present. C: MRI demonstrates persistence
of spinal cord compression by the posterior ring of the atlas despite
maximal reduction in extension. D,E: This
patient underwent occipitocervical arthrodesis with laminectomy of the
atlas and foramen magnum enlargement. Spinous process wires were used
at C-2 and C-3. The patient obtained solid arthrodesis with resolution
of spasticity.
arthritis is essentially the same as that in cervical trauma and is
discussed in Chapter 140.
-
Approach the appropriate cervical
vertebrae through a midline posterior approach. Place a Gelpy retractor
longitudinally in the incision to remove skin folds. For subaxial
approaches, obtain a lateral radiograph with a Kocher clamp placed on
an exposed spinous process
P.4005P.4006
to
determine the appropriate spinal level. A triple-wire fixation
technique is most widely applicable. If the spinous processes are
deficient, use lateral mass plates. -
Postoperatively, immobilize the patient in a Philadelphia collar for 12 weeks.
-
Pay careful attention to staying in the
midline during initial dissection. If you can see muscle fibers, the
dissection has strayed to one side. Maintaining a midline position will
limit bleeding. -
Stabilize the C-2 vertebra with a towel
clip or Kocher clamp during dissection of soft tissues to prevent gross
movements of the vertebrae. -
Do not use sublaminar wires at C-1 or C-2
if neurologic compression is present. In these cases, perform a careful
laminectomy of C-1 and use a spinous process wire at C-2. -
If a spinous process wire is used, gently
work the towel clip from side to side until it moves readily through
the hole. This technique will allow passage of the transverse wire with
relative ease.
perioperative mortality due to airway compromise, neurologic
deterioration, infection and wound problems, hardware and graft
failure, and surgical complications such as myocardial infarction,
pulmonary embolus, and pulmonary or urinary tract infection. Late
complications include nonunion and adjacent segment instability with
recurrent neurologic compromise.
significant concern after cervical spine surgery. These concerns are
magnified in the case of rheumatoid arthritis, because these patients
often have decreased pulmonary reserve and difficulties with
postoperative mobilization. For these reasons, consider preoperative
tracheostomy for patients undergoing anterior corpectomies or dens
resection and patients with severe neurologic compromise (14,27).
Alternatively, maintain these patients on a ventilator for several days
postoperatively to allow resolution of airway edema. All other patients
with rheumatoid arthritis should undergo fiberoptic intubation to
reduce postoperative airway complications (59).
concern in rheumatoid patients because of the atrophy of their skin and
soft tissues, as well as the immunosuppressive
effects
of their medication regimens. Administer intravenous antibiotics
preoperatively, and maintain patients on antibiotics until
postoperative drains have been removed. Reduction of foreign material
in the wound is also important in obtaining wound healing. While
supplementary PMMA was recommended in the past, it has been associated
with wound healing problems and is no longer used (9,13,14).
deterioration should be a rare occurrence. Interoperative spinal cord
monitoring should be used routinely to allow the earliest possible
detection of potential spinal cord injury and immediate institution of
measures with the potential to reverse neurologic compromise, such as
removal of hardware or wires. Obtain a CT scan or MRI on patients in
whom deficits develop or worsen postoperatively to rule out an epidural
hematoma and bone graft or hardware malposition. Patients with new
deficits and radiographic evidence of spinal cord compromise should
undergo an emergent wound exploration, and appropriate steps should be
taken to reverse the source of the neurologic compression.
deficits experience improvement in function with surgical decompression
and solid fusion. The long-term stability of these results, however,
has been a concern because of the potential for new subluxations caudal
to the original arthrodesis (41). While new
subluxations may partly reflect disease progression, they also may be
accelerated by the mechanical effect of the adjacent fusion.
a minimum 10-year follow-up of a series of 38 patients treated with
posterior arthrodesis. Nineteen patients died during the follow-up
period. Four patients (11%) underwent further arthrodesis for
subluxations caudal to the original procedure. Although 12 of 24
patients (50%) undergoing a Gallie (22) atlantoaxial arthrodesis developed nonunion, it did not appear to adversely affect their clinical outcome.
incidence of caudal subluxations of 79 patients treated with either
occipitocervical (24 patients) or atlantoaxial (55 patients)
arthrodesis. They found that patients undergoing occipitocervical
arthrodesis experienced a higher and more rapid rate of caudal
subluxation requiring revision surgery. Of the patients with occipital
arthrodesis, 36% developed subaxial subluxation in an average of 2.6
years, compared with 5.5% of the patients undergoing atlantoaxial
arthrodesis in an average of 9 years postoperatively.
Thirteen patients (32%) had died by the time of the later follow-up.
Eighteen patients underwent clinical and radiographic evaluation, and
nine were interviewed. None of these 27 patients had had clinical or
radiographic deterioration over the length of the follow-up.
rheumatoid patients following arthrodesis. Overall rates of nonunion
have ranged from 8% to 50% in various series of posterior cervical
arthrodesis in patients who have rheumatoid arthritis (8,14,27,28,41,43,48,49).
In series of patients with mixed diagnoses treated with uniform
surgical procedures, rates of nonunion are somewhat higher for
rheumatoid patients than for patients with other diagnoses (2,8,35,37,39,61).
While new technology may ultimately improve patient outcomes, adherence
to proven surgical techniques such as use of structural
corticocancellous autograft, good apposition of graft to bony surfaces,
and appropriate postoperative bracing lead to good results for the
majority of patients.
arthritis who are at risk of developing neurologic injury, as recovery
of neurologic function once deficits develop is uncertain and often
incomplete. New imaging modalities, as well as means of interpretation
of plain radiographs, allow more accurate selection of patients at risk
for neurologic injury. While new techniques of internal stabilization
should improve rates of achieving solid arthrodesis, an assessment of
the risks of these techniques in rheumatoid patients is needed. The
long-term effect of such fixation on adjacent motion segments is not
known. The primary determinants of satisfactory outcomes remain careful
patient selection, appropriate choice of surgical procedures, and
adherence to the principles and techniques of neurologic decompression
and spinal arthrodesis.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
F, Algra P, Vielvoye C, Cats A. Magnetic Resonance Imaging in the
Evaluation of Patients with Rheumatoid Arthritis and Subluxations of
the Cervical Spine. Arthritis Rheum 1987;30:624.
W, Inglis A, Sculco T, Ranawat C. Methylmethacrylate Stabilization for
Enhancement of Posterior Cervical Arthrodesis in Rheumatoid Arthritis. J Bone Joint Surg 1982;64:1045.
J, Fallahi S. Cervical Discovertebral Destruction, Subaxial
Subluxation, and Myelopathy in a Patient with Rheumatoid Arthritis. Arthritis Rheum 1981;944.
T, Tsuji H, Katoh Y, et al. Occipitocervical Fusion Reinforced by
Luque’s Segmental Spinal Instrumentation for Rheumatoid Diseases. Spine 1988;13:1234.
EKM, Ketonen L, Sepponen R, et al. Low-field MRI in the Evaluation of
Rheumatoid Cervical Spine. Comparison with Neurological Findings and
Routine Plain Radiography. Clin Exp Rheumatol 1990;8:365.
D, Peppelman W, Agarwal A, et al. Incidence of Subaxial Subluxation in
Patients with Generalized Rheumatoid Arthritis Who Have Had Previous
Occipital Cervical Fusions. Spine 1991;16:S486.
A, Refior H, Westermann S. The Importance of Functional Magnetic
Resonance Imaging (MRI) in the Planning of Stabilizing Operations on
the Cervical Spine in Rheumatoid Patients. Arch Orthop Trauma Surg 1989;109:30.
A, Casey A, Solanki G, et al. Radiological and Anatomical Evaluation of
the atlantoaxial Transarticular Screw Fixation Technique. J Neurosurg 1997;86:961.
F, Seemann P. Stable Posterior Fusion of the Atlas and Axis by
Transarticular Screw Fixation. In: Kehr P, Weidner A, eds. Cerv Spine Wien: Springer-Verlag, 1986;322.
P, Ranawat C, Tsairis P, Bryan W. A Prospective Study of the
Progression of Rheumatoid Arthritis of the Cervical Spine. J Bone Joint Surg Am 1981;63:342.
W, Kraus D, Donaldson W, Agarwal A. Cervical Spine Surgery in
Rheumatoid Arthritis: Improvement of Neurologic Deficit After Cervical
Spine Fusion. Spine 1993;18:2375.
M, Kotzar G, Yoo J, Bohlman H. A Biomechanical Analysis of atlantoaxial
Stabilization Methods Using a Bovine Model: C1/C2 Fixation Analysis. Clin Orthop 1993;290:285.
J, Pickard J, Wood S, Prouse P. Case Report: Reversible Cortical
Blindness as a Complication of Rheumatoid Arthritis of the Cervical
Spine. Br J Rheumatol 1990;29:228.
I, Concepcion M, Hibberd P, Lipson S. Upper-Airway Obstruction and
Perioperative Management of the Airway in Patients Managed with
Posterior Operations on the Cervical Spine for Rheumatoid Arthritis. J Bone Joint Surg Am 1994;76:360.
J, Cooke D, Brook A, Corbett M. A Prospective Study of the Radiological
Changes in the Cervical Spine in early Rheumatoid Disease. J Rheum Dis 1981;40: 109.
J, Young A, Williams P, Corbett M. Prospective Study of the
Radiological Changes in Hands, Feet and Cervical Spine in Adult
Rheumatoid Disease. Ann Rheum Dis 1983;42:613.
N, Lauryssen C. Vertebral Artery Injury in C1-2 Transarticular Screw
Fixation: Results of a Survey of the AANS/CNS Section on Disorders of
the Spine and Peripheral Nerves. J Neurosurg 1998;88:634.

