COMPRESSION NEUROPATHIES OF THE UPPER EXTREMITY
III – THE HAND > Conditions of Nerves > CHAPTER 52 – COMPRESSION
NEUROPATHIES OF THE UPPER EXTREMITY
Limited Compliance, Such As The Carpal, Cubital, Or Ulnar Tunnels, or
It Lies Deep To Fibrous Bands And Tendinous Arches Of Origin, It Is
Vulnerable To Compression. An Increase In The Volume Of Material Within
One Of These Limited Spaces Or A Decrease In Size Of The Space Can Lead
To Increased Pressures, Which In Turn Can Compress The Nerve. Other
Factors That May Lead To A Mechanical Peripheral Neuropathy Include
Stretching Of The Nerve And Abnormal Nerve Motion About Or Adherence To
Fibrous Bands Or Fascial Edges. The Intrinsic Response Of The Nerve To
This Mechanical Insult Varies Little Regardless Of The Location Of The
Injury Or The
nature
of the offending agent. The sequence of pathologic changes within the
median nerve and the resulting clinical progression are the same
whether the entrapment is secondary to synovitis within the carpal
tunnel or secondary to a bone fragment from a fracture of the distal
radius. Relief of the compression, generally accomplished by releasing
the confining tunnel or compressive fibrous band, reverses these
changes partially or completely. If damage to the nerves is
irreversible (i.e., if entrapment is chronic), release can halt
progression. All three major nerves of the upper extremity, the median,
ulnar, and radial, may be injured by compression in locations where
they are anatomically vulnerable.
and function. The severity of the resulting lesion depends on the
magnitude of the compression as well as its duration. Both direct
mechanical factors causing myelin damage and alterations in blood flow
to and within the nerve likely play roles of varying degrees in causing
the nerve changes seen in compression neuropathies. Ischemic changes
caused by blood-flow alterations play a part in acute, easily
reversible nerve-function alterations (72).
Experiments have shown that elevation of pressure in and about the
nerve to within 40 mm Hg of diastolic blood pressure cause profound
changes in sensory nerve function of the median nerve, which are
reversible with restoration of blood flow. Motor dysfunction of the
nerve requires higher and more sustained elevations in pressure. These
changes in function are not associated with structural changes in the
nerve (132). Ischemia also plays a role in
chronic compression. It is believed to contribute to intraneural
scarring as well as edema from prolonged loss of blood supply to the
nerve. Structural changes, particularly alterations in or loss of the
myelin coatings of nerve fibers, are seen in chronic, higher-pressure
compressions, especially those involving an edge such as a fibrous band
or tendon. These changes resolve only in some cases, after enough time
has elapsed for repair of damaged myelin. Compression of the nerve has
also been shown to inhibit axoplasmic flow, both antegrade and
retrograde, diminishing nerve function and contributing to the bulging
appearance of the nerve proximal and distal to the site of compression (72).
not entirely understood. The frequently dramatic response to treatment
can be explained only by the reversal of a vascular lesion (33,34).
According to this theory, the mechanical factor responsible for
producing the compression obstructs venous return, followed by
segmental anoxia, capillary vasodilatation, and edema (33,125). The nerve edema aggravates the compression and leads to abnormal axonal and cellular exchange (34,124,131).
Surgical release at this stage is a rewarding procedure. Prolonged
compression results in intraneural fibrosis, after which nerve recovery
is less likely to occur despite decompression.
determination of the site of compression are greatly aided by a
knowledge of the anatomic distribution of a nerve and its function.
Clinical evaluation of nerve compression neuropathies includes sensory
threshold testing, provocative testing, and evaluation of muscle
weakness or atrophy. The most consistent and reliable way to evaluate
sensibility in nerve compression is to use threshold testing (126,127,132,133,134). Threshold tests evaluate how well a single nerve fiber innervating a receptor or group of receptor cells is functioning.
These include vibrometry, Semmes–Weinstein monofilaments, and vibration
testing. To test with Semmes–Weinstein monofilaments, apply pressure to
the fingertip with the filament until the filament bends (Fig. 52.1).
The pressure required to bend the filament is directly related to its
diameter. Apply filaments of successively increasing diameter to
determine the sensory threshold of slowly adapting nerve fibers.
Perform vibration testing with a 256 cycles-per-second (cps) tuning
fork to evaluate the sensory threshold of quickly adapting nerve fibers
(Fig. 52.2).
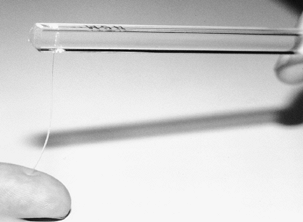 |
|
Figure 52.1. Threshold testing with Semmes–Weinstein monofilaments.
|
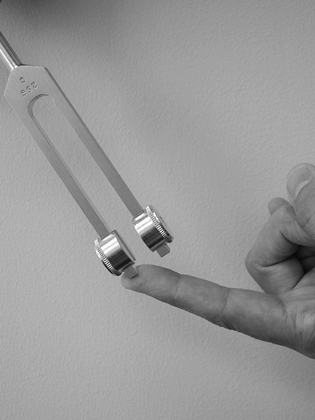 |
|
Figure 52.2. Vibration sensation testing with a 256 cps tuning fork.
|
and correctly represented in the cerebral cortex. Early in compression
neuropathies, nerve fibers are not lost; rather, the nerve fibers that
are present are not functioning well. In nerve laceration, in contrast,
nerve fibers to the innervated
area
are lost. Innervation density tests are more useful for evaluating
nerve laceration and recovery after repair than for evaluating
compression neuropathies. These tests are abnormal in only 20% of
patients with electrically proven carpal tunnel syndrome (CTS), whereas
threshold tests detect more than 80% of electrically proven CTS
patients (133).
 |
|
Figure 52.3. Two-point discrimination testing.
|
nerve to elicit numbness and paresthesias in its sensory distribution.
Provocative tests are especially important in patients with exertional
compression neuropathies, where symptoms and signs may be minimal or
absent at rest. [For this reason, it may also be advisable to perform
sensory testing before and after activity (12).]
The examiner evaluates weakness and atrophy subjectively. Muscles
innervated by the nerve suspected of compression are tested for bulk
and strength. Comparison to the uninvolved extremity is especially
helpful.
examination, electrodiagnostic studies may help to corroborate clinical
impressions. They truly are the only objective test of nerve
compression. Additionally, they may indicate a level of severity of
nerve damage that may have prognostic implications for treatment. A
detailed explanation of neurodiagnostics of the upper extremity is
beyond the scope of this chapter. Here we offer a brief, basic
description of methods and definitions of terms. For a more complete
understanding, read review articles and book chapters published on this
subject (49,51,56).
The neurodiagnostic study, when used for the majority of upper
extremity compression injuries, is composed of two parts: the nerve
conduction or velocity (NCV) and the electromyography (EMG) needle
examination. The NCV measures the speed of conduction of an impulse
along a segment of nerve. The EMG measures the response of muscle
fibers to conducted nerve impulses.
propagates an electrical impulse. This impulse can be detected by a
surface electrode on the skin overlying a nerve or a muscle innervated
by a nerve. In an NCV test, the nerve is stimulated electrically at one
point and the impulse measured at another point along its length. When
the study measures an impulse traveling in a physiologic direction, it
is called an orthodromic study; when it measures an impulse traveling
in the opposite direction, it is called antidromic. Impulses propagated
by sensory nerves or the sensory fibers of a mixed nerve are measured
by surface electrodes as a waveform called the sensory nerve action
potential. Motor nerves or the motor fibers of mixed nerves are
measured by the electrical response of multiple muscle fibers, which
produce a waveform known as the composite muscle action potential.
at which the sensing electrode detects the waveform is known as the
latency. In motor nerves, latency also encompasses the time it takes
the nerve impulse to be transmitted across the neuromuscular junction
and to activate the muscle fibers. Neurodiagnosticians often report
latencies, especially when evaluating nerve conduction across short
nerve segments at the wrist. Latencies are reported in units of time
(msec). Conduction velocities are reported in units of meters per
second and can be calculated from the measured latency and knowledge of
the length of the nerve segment over which the latency was measured.
Velocities are reported for longer lengths of nerve segments tested,
such as from the midforearm to the hand. Additional measurements can be
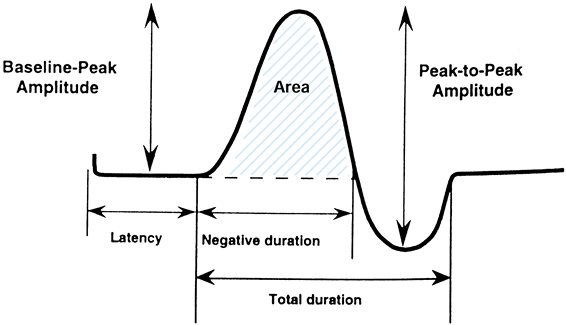
obtained by evaluating the waveforms. These include the amplitude,
duration, and area of the waveform (Fig. 52.4).
These parameters can aid the electromyographer in characterizing the
conduction of the nerve. The amplitude and duration depend on the
number of nerve fibers and the speed of conduction of the different
nerve fibers transmitting the stimulated impulse, respectively.
 |
|
Figure 52.4.
A waveform obtained from nerve conduction testing. (From Holmlund T. Electrodiagnosis and Neurologic Evaluation. In: Peimer CA, ed. Surgery of the Hand and Upper Extremity. New York: McGraw-Hill, 1996:1277, with permission.) |
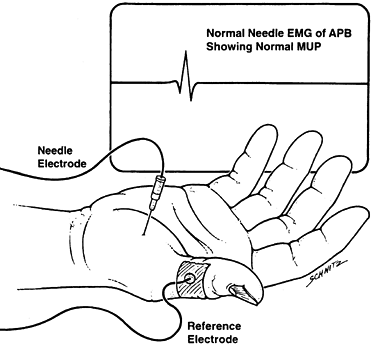
muscle to evaluate the activity of a single motor unit consisting of
the nerve cell, its fibers, and the group of muscle fibers it
innervates (Fig. 52.5). Normally, a muscle
fiber at rest is essentially electrically silent. After a brief burst
of electrical activity that occurs while the electrode is inserted, the
needle measures only occasional background impulses. When the muscle is
voluntarily contracted, the electrode senses the electrical activity of
the motor unit, which is a waveform called the motor unit potential
(MUP). In the pathologic state, different waveforms may be detected
that give insight into the disease process affecting the muscle as well
as the chronicity of the condition and whether recovery or further loss
is occurring. (Some names of these waveforms and their general
implications are offered here, but this information should not be
considered a definitive reference.)
 |
|
Figure 52.5.
Electromyography (EMG). APB, abductor pollicis brevis; MUP, motor unit potential. (From Hillburn JW. General Principles and Use of Electrodiagnostic Studies in Carpal and Cubital Tunnel Syndromes. Hand Clin 1996;12:205, with permission.) |
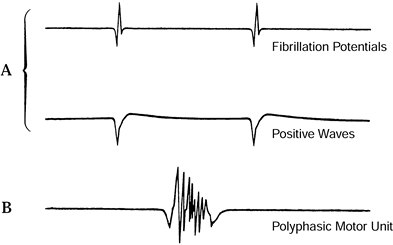
The development of small highly polyphasic MUPs (Fig. 52.6B)
and decreased fibrillations is considered evidence of early
reinnervation of muscle. MUPs that are of great duration and amplitude
are considered evidence of chronic denervation with collateral
reinnervation resulting from adjacent nerve sprouting. Many waveforms
are characteristic of neuropathies, and others of myopathies. The
significance of the different electrical events is subject to the
clinical setting and the interpretation of the electromyographer.
 |
|
Figure 52.6. Abnormal EMG motor unit potentials. A: Fibrillation potentials and positive sharp waves indicate recent muscle denervation. B:
Polyphasic MUPs indicate early reinnervation of muscle. (From Hillburn JW. General Principles and Use of Electrodiagnostic Studies in Carpal and Cubital Tunnel Syndromes. Hand Clin 1996;12:205, with permission.) |
important to understand that “normals” for particular tests are
laboratory, machine, and operator dependent because of variations in
how measurements are calculated, technique, and environmental factors
such as skin temperature. However, rule-of-thumb normals may be useful.
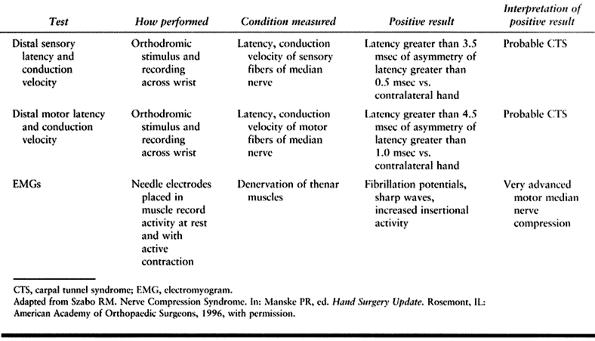
For the median nerve at the wrist, distal motor latencies of more than
4.5 msec and distal sensory latencies of more than 3.5 msec are
considered abnormal. In a patient with unilateral involvement, a
difference from one hand to the other of more than 1.0 msec for motor
latency and 0.5 msec for sensory latency is also considered abnormal (Table 52.1) (126). It is helpful to develop a relationship with an electromyographer who produces reliable and
consistent results and whose interpretations of these studies are useful in your evaluation and treatment of patients.
 |
|
Table 52.1. Electrodiagnostic Tests for Carpal Tunnel Syndrome
|
nerve function. Conditions commonly associated with nerve dysfunction
include hypothyroidism with myxedema (98), obesity (84,85), cervical radiculopathy, diabetes mellitus, alcoholism (83),
and exposure to neurotoxic chemicals or chemotherapeutic agents. These
disorders in and of themselves can cause polyneuropathy, independent of
compression neuropathy; they may increase the susceptibility of nerves
to compression; or they may negatively affect recovery of nerves after
adequate treatment. When suspected, these disorders should be
investigated and managed. They are not, however, contraindications to
treatment of coexisting compression neuropathies.
popular media, and in the medicolegal and workers’ compensation arena
have included certain compressive neuropathies, most notably CTS, in a
category of disorders known as cumulative trauma disorders or
repetitive stress injuries. The association between repetitive stress
or activity, and compression neuropathies has been described (41).
It is fairly well accepted that certain activities or prolonged
positioning of the extremities can incite the symptoms of compression
neuropathies, but controversy exists about whether certain activities
or occupations can cause them (129).
compression neuropathies of the upper extremity is an accurate
diagnosis. A perfectly performed surgical procedure is likely to
provide no benefit to the patient if it is not indicated for the
condition causing the symptoms. Remember that each of the compression
neuropathies is related to a syndrome, which is a constellation of
symptoms and signs (123). Positive
neurodiagnostic studies in the absence of symptoms and signs cannot
make the diagnosis of any of the compression neuropathy syndromes.
their compression neuropathies may be helpful in determining treatment
options. Mild cases are those with a short history of symptoms that are
intermittent rather than continuous, and with negative or minimally
abnormal electrodiagnostic findings. Severe cases are those with
histories of symptoms for more than a year, profound and persistent
numbness with atrophy of involved musculature, and both very prolonged
(or absent) conduction velocities and advanced EMG findings. Mild cases
are likely to respond to nonoperative measures (40), whereas severe cases do not, and therefore initial treatment should be operative.
physiotherapy, corticosteroid injections, and correction of metabolic
abnormalities. The nerve may be protected through avoidance of
positions that are deleterious to nerve function. For instance,
prolonged extreme positioning of the wrist in flexion in patients with
CTS or of the elbow in flexion in patients with cubital tunnel syndrome
aggravates symptoms and should be avoided. Splinting is employed,
usually at night, to prevent this type of positioning. Therefore, nerve
protection involves educating the patient about activities and postures
that may lead to compression of nerves at vulnerable sites, and how to
avoid them.
anti-inflammatory drugs (NSAIDs) and diuretics, have been used in
treating compression neuropathies. No medications, however, have any
documented efficacy. Physiotherapy may be helpful in treating certain
types of nerve compression but is most helpful in postoperative
rehabilitation. Corticosteroid injection is usually employed after
failure of the previously described nonoperative treatments. A powerful
anti-inflammatory agent is delivered directly to the tissues about the
nerve, at a specific location. Since few cases of CTS are the result of
acute or chronic inflammation in the flexor tenosynovium (62,82),
the mechanism whereby steroid injections relieve symptoms is unknown.
In addition to their therapeutic value, injections may help in
confirming a diagnosis, providing a prognostic indicator of the
potential effectiveness of operative treatment (45).
gloves at night until delivery of the child, when it will likely
resolve spontaneously. For severe symptoms, local injections of
steroids may help. If symptoms persist thereafter, operation may be
necessary.
management; acute, rapidly progressive involvement; severe cases; and
symptom recurrence. Tourniquet control during surgery is preferred
unless the site of compression is too proximal or unless specific
contraindications exist. Meticulous hemostasis is imperative during the
exposure, especially in patients with a coagulopathy and in those who
are taking aspirin or are on anticoagulation treatment to reduce
postoperative bleeding and swelling. Facilitate this by exsanguinating
the extremity through elevation rather than by elastic wrapping, so
that vessels remain partially filled and are easier to identify and
coagulate or tie off.
may be performed under general, regional, or local anesthesia. Local
anesthesia is reserved for less involved cases and can be used with
tourniquet control, which is well tolerated for brief procedures. Make
the incision to allow adequate visualization of the nerve and all
suspected sites of compression. The choice of treatment of the nerve
depends
on its appearance under direct visualization, the location of the
entrapment, and the clinical findings. If the bed in which the nerve
lies is scarified or makes the nerve vulnerable to potential mechanical
trauma, consider nerve transposition or flap coverage.
the carpal tunnel or deep to the origin of the pronator teres (PT). A
third form of median nerve entrapment is isolated compression of the
anterior interosseous nerve (AIN).
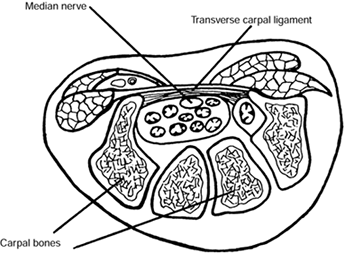
the confined space of the carpal tunnel along with the long flexor
tendons to the fingers and thumb. The tunnel is bounded on three sides
by the bones of the carpus, which make up the floor of the canal, and
on the fourth by the transverse carpal ligament (TCL), which forms the
roof (Fig. 52.7). Compressive neuropathy of the
median nerve within the carpal tunnel may result from any
space-occupying lesion under the TCL (19,67,145).
A frequent cause is flexor tenosynovitis; other causes are fractures
and dislocations of the floor of the canal and distal radius, and other
space-occupying lesions such as tumors and ganglia. These
space-occupying lesions increase the volume of the contents of the
noncompliant carpal tunnel, raising the pressure on its contents, which
include the median nerve. In many cases, there are no particular
identifiable causes even though the nerve is clearly compressed.
Although many of these cases are attributed to “nonspecific synovitis,”
pathologic examination of the synovium obtained from the carpal canal
in these cases usually fails to reveal signs of inflammation. Rather,
fibrosis and/or edema changes are seen, which may themselves be
secondary to compression rather than the primary cause of the
entrapment neuropathy (62,82).
 |
|
Figure 52.7.
Cross section of a wrist through the carpal canal. The median nerve and digital flexor tendons lie in the space formed by the bony carpal arch and transverse carpal ligament. (Adapted from North ER, Kaul MP. Compression Neuropathies: Median. In: Peimer CA, ed. Surgery of the Hand and Upper Extremity. New York: McGraw-Hill, 1996:1307, with permission.) |
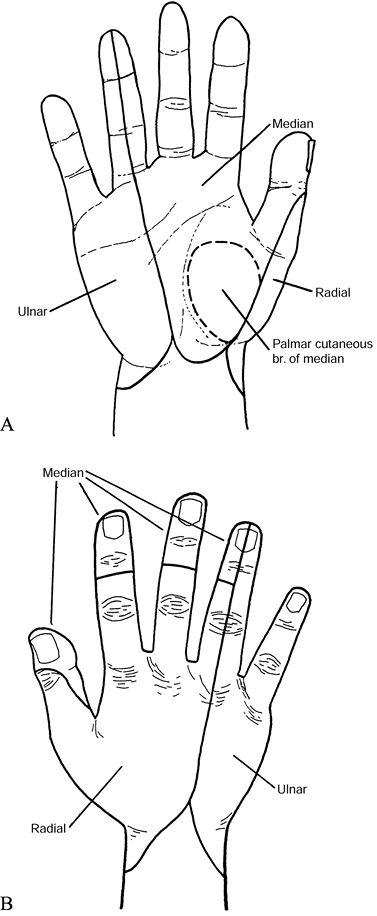
patient’s history. Typically, he complains of aching or burning pain
along the median nerve distribution and of numbness and tingling in the
median-nerve-innervated digits (Fig. 52.8A)
during the night and early morning as well as during activities.
Numbness may extend into the ulnar digits in some patients. These
symptoms are aggravated by elevation, repetitive activities, and
prolonged flexion positioning of the wrist (130).
Radiation of symptoms proximal to the wrist is not unusual. Complaints
of the hand feeling fat, clumsiness in manipulation, and dropping items
are also frequent. The incidence is greater in women than in men,
although the difference is decreasing. In the past, postmenopausal
women were the most common patients; commonly associated diagnoses were
rheumatoid arthritis and distal radius malunion. Recently, a large,
younger group of patients with essentially equal distribution of women
and men has emerged. In this group the carpal tunnel disease has been
labeled idiopathic (62).
 |
|
Figure 52.8. A: Palmar sensory distributions of ulnar, median, palmar cutaneous, and radial nerves. B: Dorsal sensory distributions of radial, median, and ulnar nerves.
|
and strength testing. Sensibility may be reduced throughout the area
normally supplied by the median nerve except for the thenar eminence,
the distribution of the palmar cutaneous branch (Fig. 52.8A), which does not
enter the carpal canal (135).
As noted previously, the most consistent and reliable way to evaluate
sensibility in nerve compression is to use threshold testing
(Semmes–Weinstein monofilaments, vibrometry, and 256 cps vibration
testing) (126,127,132,133,134).
Provocative tests compress or percuss the median nerve to elicit
numbness and paresthesias in the distribution of the median nerve in
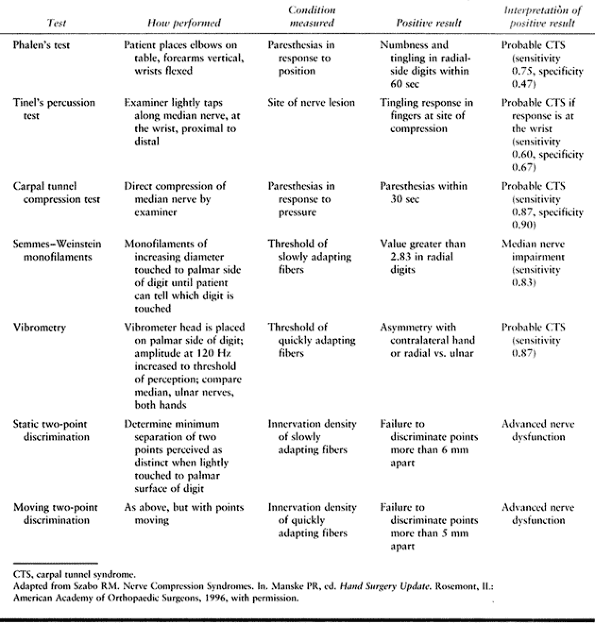
patients with CTS. Phalen’s wrist flexion test, in which the wrist is
maximally flexed with the fingers slightly curled, is sensitive (Fig. 52.9) (43).
A positive test for CTS is reproduction of symptoms within 60 sec.
Tinel’s nerve percussion test, in which the median nerve is percussed
as it enters the carpal canal to elicit symptoms (Fig. 52.10), is specific and indicates CTS in cases in which Phalen’s test is also positive (65). Another useful test is the direct compression test, which is sensitive and specific. The examiner’s thumbs apply direct pressure to the median nerve as it enters the carpal tunnel (Fig. 52.11) (29).
A positive test is reproduction of symptoms, which appear within 30 sec
and disappear with release of compression. Together, these tests
provide added clinical evidence of median nerve compression at the
wrist (Table 52.2).
 |
|
Figure 52.9. Phalen’s wrist flexion test.
|
 |
|
Figure 52.10. Tinel’s nerve percussion test.
|
 |
|
Figure 52.11. Durkan’s median nerve compression test.
|
 |
|
Table 52.2. Diagnostic Tests for Carpal Tunnel Syndrome
|
a pen) between the patient’s fingers, which are held together. The
object slides much more easily on dry skin than on skin with normal
perspiration. Strength testing is difficult and somewhat subjective.
The most easily evaluated muscle of the thenar eminence is the abductor
pollicis brevis (APB) muscle. Most often, this muscle is innervated
solely by the median nerve, but it can also have a contribution from
the radial nerve. It is the most superficial of the thenar muscles and
can be palpated during active opposition or resisted palmar abduction
of the thumb. Relative weakness of palmar abduction of the thumb
against resistance or muscle atrophy occurs in more advanced cases.
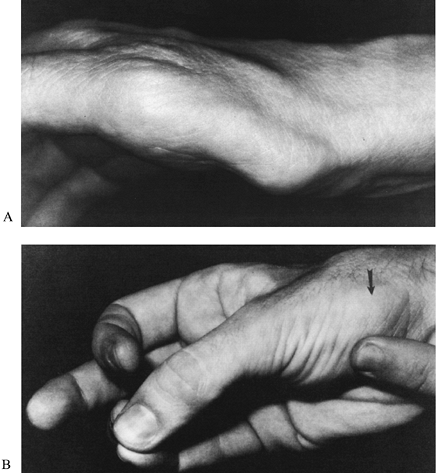
Flattening or concavity of the normally bulging thenar eminence indicates atrophy of the APB (Fig. 52.12A). A weak, soft, or small APB is seen in severe cases of CTS and indicates denervation of the muscle.
 |
|
Figure 52.12. A: A flattened thenar eminence indicates atrophy of the abductor pollicis brevis. B: Abductor pollicis brevis (arrow). (From North ER, Kaul MP. Compression Neuropathies: Median. In: Peimer CA, ed. Surgery of the Hand and Upper Extremity. New York: McGraw-Hill, 1996:1307, with permission.)
|
it should be restricted to patients with a history of trauma or
arthritis and those with decreased wrist range of motion on
examination. One of us (JT) obtains radiographs to rule out Kienböck’s
disease in younger patients and peritrapezial arthritis in older ones.
Additional diagnoses uncovered by preoperative radiographs include
scapholunate advanced collapse (SLAC) wrists, ununited fractures of the
scaphoid, and radiopaque masses within the carpal tunnel (136). The views recommended include posteroanterior (PA), lateral, and carpal tunnel views.
studies and laboratory tests. Positive nerve conduction velocities show
increased latencies. In severe cases, EMG exams may show abnormalities
and may give information regarding treatment prognosis. In mild or
exertion-related cases, electrodiagnostic studies may be negative. This
negative finding does not rule out CTS in the presence of typical signs
and symptoms. Provocative nerve conduction evaluation may help to
uncover these dynamic forms of CTS (14). EMG
examination may be helpful when a cervical radiculopathy is suspected.
Order laboratory testing to screen for metabolic disorders that may
contribute to or cause CTS, (a) when there is suspicion of these
disorders, (b) when there is bilateral presentation, and (c) in
children who may have rare mucopolysaccharidosis or mucopolylipidosis (139).
Tests include erythrocyte sedimentation rate, rheumatoid factor, serum
glucose level, uric acid, thyroid panel, and renal indices. Liver
function tests may be indicated if alcohol-related peripheral
neuropathy is suspected. If doubt persists about the correct diagnosis,
an injection of a small amount of a steroid preparation mixed with
local anesthetic into the carpal
tunnel (not into the nerve) can be a therapeutic and diagnostic aid (Fig. 52.13) (Table 52.3) (45).
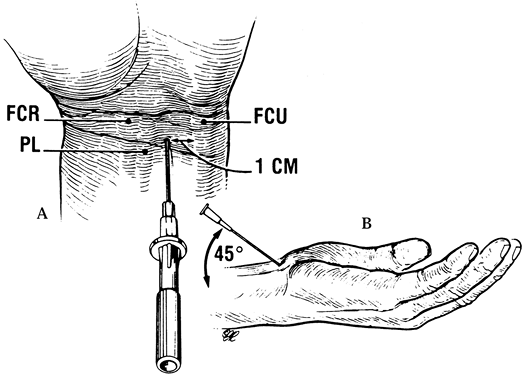
The entry site for the needle is slightly proximal to the distal wrist
crease, ulnar to the palmaris longus (PL) to avoid impaling the median
nerve, and approximately 1 cm radial to the flexor carpi ulnaris (FCU)
to avoid entrance into the canal of Guyon. Insert the needle at a 45°
angle, beneath the proximal margin of the TCL and directed in line with
the ring finger ray. Palpation in the midpalm just distal to the TCL
while injecting can help confirm proper needle placement by enabling
you to feel the flush of fluid into the canal.
 |
|
Figure 52.13. Injection into the carpal tunnel. A: The entry site for the needle. PL, palmaris; FCU, flexor carpi ulnaris. B:
Insertion of the needle. (From Gelberman RH, Rydevik BL, Pess GM, et al. Carpal Tunnel Syndrome: A Scientific Basis for Clinical Care. Orthop Clin North Am 1988;19:117, with permission.) |
 |
|
Table 52.3. Carpal Tunnel Syndrome Summary
|
categories. The mild group consists of patients with intermittent
symptoms that have been present less than 1 year, who have normal
two-point discrimination, no thenar weakness or atrophy, no denervation
potentials on EMG, and mildly elevated NCV. With conservative treatment
and steroid injection, 40% will be free of symptoms at 12 months. The
severe group consists of those with profound, persistent symptoms that
have been present longer than 1 year, thenar weakness or atrophy, and
marked abnormalities on electrodiagnostic studies (40).
Patients in the severe group fail to respond adequately to conservative
therapy and should receive operative treatment, which may include
tendon transfers concurrent with carpal tunnel release. In the moderate
group, conservative treatment shows findings and gives results
intermediate between those of the mild and severe groups. The presence
of underlying disorders or advanced age in any of these patients
diminishes the response to conservative (and possibly operative) care.
patients except those with severe disease. Splint the wrist in neutral
to slight extension at night and during activities that exacerbate
symptoms. In exertional or dynamic cases, modification of activities
that exacerbate symptoms may be helpful. Vitamin B6 has no effect on the natural history of CTS (3,4).
Steroid injection offers transitory relief in 80% of patients, with
only 22% being free of symptoms at 12 months. The patients in the mild
group fare better than others with steroid injection (40). Treat or correct underlying disorders if possible.
treatment, persistent or progressive symptoms, acute onset with
profound sensory loss associated with trauma, and weakness or atrophy
of thenar muscles. Results of surgery vary according to severity of
disease, choice of surgical treatment, and individual patient
physiology and social issues. As a general rule, we believe that with
adequate release of the carpal tunnel, major or complete relief of
discomfort associated with CTS is likely, as is relief of transient
numbness. Persistent or profound numbness may not disappear with
release if it has been present for a prolonged period (over 12 months).
Weak or atrophied
muscle
is not expected to recover with release, but if it is not complicated
by underlying disease, its progression will likely be halted. Nolan et
al. (87)
showed that patients with severe disease, followed for more than 2
years after carpal tunnel release, show improvement. Therefore, surgery
is indicated in these patients, especially if symptoms of discomfort
are present.
patient with CTS in the context of the patient’s general condition. You
must be certain of the diagnosis of CTS and that its cause is
compression of the median nerve at the wrist. Within reason, exclude
other potential sources of the symptoms and maximally correct any
contributing conditions. With these issues resolved, you must include
the particular considerations of the patient in the surgical care. If
malunions and carpal instabilities are severe enough to compromise
results, treat them prior to or concurrently with release. In patients
with severe atrophy of the thenar musculature, perform opponensplasty
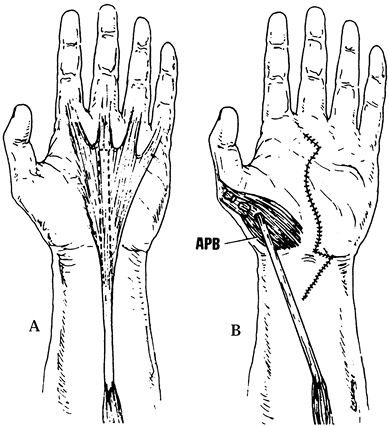
at the time of release. A particularly suitable procedure for
restoration of thumb palmar abduction in the patient with severe CTS is
the Camitz opponensplasty, in which the PL is harvested and extended
with a strip of palmar fascia, then routed subcutaneously and sutured
into the thumb at the level of the metacarpophalangeal (MP) joint along
the APB tendon (Fig. 52.14).
 |
|
Figure 52.14. The Camitz transfer for restoration of thumb palmar abduction in patients with severe atrophy of the thenar musculature. A: Harvesting of the palmaris longus. B: Rerouting of the PL. (From Imbriglia JE, Hagberg WC, Baratz ME. Median Nerve Reconstruction. In: Peimer CA, ed. Surgery of the Hand and Upper Extremity. New York: McGraw-Hill, 1996:1381, with permission.)
|
-
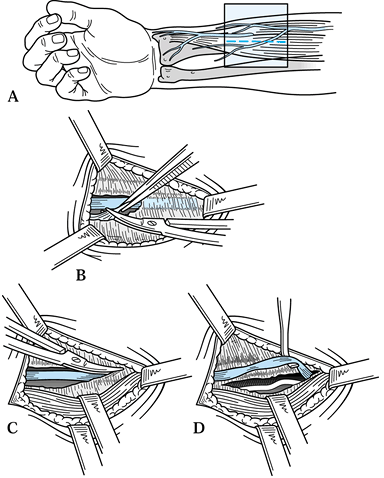
Start the incision at the junction of the
proximal and middle thirds of the palm (at the level of Kaplan’s
Cardinal Line) on the radial aspect of the ring finger ray and
P.1558
continue it proximally to a point where the interthenar crease intersects the distal wrist crease, just ulnar to the PL tendon (Fig. 52.15).![]() Figure 52.15. Approach to the carpal tunnel. The more proximal porton (dashed and dotted lines) is used when a more extensive exposure is required.
Figure 52.15. Approach to the carpal tunnel. The more proximal porton (dashed and dotted lines) is used when a more extensive exposure is required. -
To extend this incision for increased
exposure, as is needed in flexor tenosynovectomy, carry the incision
along the wrist crease ulnarly to a point just radial to the flexor
carpi ulnaris tendon and, if necessary, gently curve it proximally and
radially to the ulnar aspect of the PL tendon. -
Divide the subcutaneous fat to expose the
palmar fascia and divide the palmar fascia in line with the incision,
which exposes the TCL. Take care to avoid injury to the ulnar nerve and
artery that lie nearby, on the ulnar aspect of the field in the canal
of Guyon. -
Divide the TCL longitudinally, close to
its ulnar attachments and extending distally until you reach the fatty
envelope surrounding the vessels of the superficial palmar arch. -
Proximally, with the median nerve under
direct vision, elevate the skin off of the TCL where it blends into the
antebrachial fascia. -
Divide the proximal portion of the TCL and the antebrachial fascia with scissors 3–5 cm proximal to the carpal canal.
-
Elevate the entire radial flap of skin,
including subcutaneous tissue, ligament, and intact palmar cutaneous
branch of the median nerve and its divisions, to expose the median
nerve in the canal. -
Use blunt dissection to free the median nerve from the radial wall of the canal, where it is often tethered by synovium.
-
Sweep the contents of the canal away from
first the radial and then the ulnar aspects of the canal, inspecting
the floor for masses or irregularities. Also examine the digital flexor
tendons for fraying and other damage. -
Perform synovectomy (e.g., in rheumatoid patients), and reduce and fix fractures or dislocations if indicated.
-
Release the tourniquet and obtain hemostasis. Close only the skin with 5-0 nylon suture.
-
Apply a well-padded dressing with plaster
reinforcement as desired to immobilize the wrist in neutral and the
thumb in palmar abduction. Be sure not to splint the thumb in radial
abduction because this position encourages lengthening of the thenar
muscles, which results in prolonged pinch weakness.
whether internal neurolysis improves the results of carpal tunnel
release in patients with severe CTS manifested by thenar atrophy or
fixed sensory deficit (33,34,42,71,73).
In a combined series of patients (69 hands) from San Diego and
Sacramento, added benefit from internal neurolysis in the treatment of
severe CTS could not be demonstrated (42). Lowry and Follender (71)
confirmed this finding with a prospective, randomized, double-blind,
controlled study. We therefore no longer perform or recommend internal
neurolysis in the treatment of CTS. Concomitant release of the canal of
Guyon in patients with evidence of compression of the ulnar nerve at
the level of the wrist in conjunction with CTS is not recommended (117).
Transection of the TCL during carpal tunnel release alone results in an
increase in the volume of the carpal tunnel of approximately 24% and a
change in the orientation and shape of the canal of Guyon (102). Clinically, this results in resolution of the symptoms referable to ulnar nerve compression at the wrist in these patients.
surgical technique, especially for surgeons not doing a large volume of
these surgeries, because it offers good visualization of the TCL, the
superficial arch, and the carpal canal contents. It is a proven,
reliable method. Nonetheless, some herald endoscopic carpal tunnel
release as a step forward in the treatment of CTS (15,21,94).
Proponents of the latter technique claim decreased morbidity as a
result of the smaller scar, which occurs away from the base of the
palm, and more rapid rehabilitation (35,142).
Several systems exist for the purpose of endoscopic release; they have
in common visualization of the undersurface of the TCL with an
arthroscope as the ligament is divided from within the canal using
special knives or a blade mechanism. Agee et al. (2) and Chow (21) developed independent systems that are available commercially.
demonstrated a decrease in early postoperative pain and morbidity using
his device, but by 6 weeks, results were comparable to those of
standard carpal tunnel release.
Although scar pain has been reduced and time to recovery of
preoperative grip and pinch strength minimally shortened, pillar pain
and palmar tenderness have not been eliminated. In cadaver studies,
incomplete release of the TCL has been demonstrated to occur in up to
50% of the specimens (111). Clinically,
however, patients do obtain symptomatic relief from the endoscopic
technique. As endoscopic release systems have been refined and
experience with the technique gained, results have improved. The
procedure remains hazardous in inexperienced hands and requires
specialized training. A risk of cutting neurovascular structures or
tendons exists because of limited visualization and deviations from
proper technique (81,110).
Recurrent or incomplete relief of symptoms from endoscopic release has
been shown to be related to incomplete release of the TCL. This
improves after subsequent open carpal tunnel release (36,53).
method for treatment of CTS. However, with a thorough knowledge of the
pertinent anatomy and with experience, it can be a safe and effective
treatment in appropriate patients if the surgeon adheres to several
tenets. The surgical consent form must specify both an endoscopic and
an open carpal tunnel release, because several conditions may
necessitate aborting the former and proceeding with the latter. These
include (a) difficulty in visualization that results from equipment
problems, fogging, or the inability to clear synovium from the
undersurface of the ligament, (b) presentation of atypical anatomy, and
(c) difficulty in manipulating the endoscope. The surgeon must place
the endoscope in the ulnar aspect of the carpal tunnel and keep it
aligned with the ring finger ray to prevent injury to the median nerve
and the superficial vascular arch (108).
(Specific techniques are not discussed here; refer to the
manufacturer’s technique manual for each system.) The surgeon must
verify absolutely that the endoscope is within the carpal tunnel and
not in the canal of Guyon before making any cuts. Endoscopic carpal
tunnel release is inappropriate in patients with bony deformity caused
by fracture or dislocation and in those with inflammatory synovitis who
require synovectomy or whose synovitis may make visualization
difficult. If endoscopic tunnel release is considered, preoperative
radiographs are necessary to evaluate these issues.
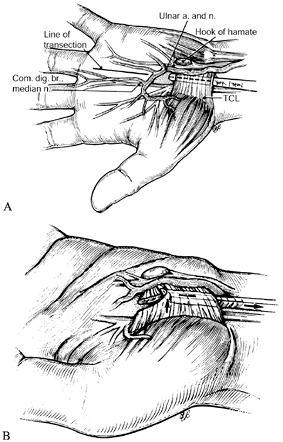
systems have been developed for carpal tunnel release and are
commercially available (13,68,86).
These differ from endoscopic techniques in that the TCL is divided by a
relatively blind method rather than being visualized with an
arthroscope. The benefits of this technique over open carpal tunnel
release are similar to those of the endoscopic methods, as are the
risks.
 |
|
Figure 52.16. Endoscopic carpal tunnel release with the system developed by John M. Agee. A: Insertion of the blade assembly beneath the transverse carpal ligament. B: Division of the transverse carpal ligament. (From Gelberman RH, North ER. Carpal Tunnel Release. In: Gelberman RH, ed. Operative Nerve Repair and Reconstruction. Philadelphia: Lippincott, 1991:899, with permission.)
|
mobility of the elbow and shoulder. Elevation is crucial, especially
during sleep, to reduce edema. Remove bulky dressings and splints 5
days postoperatively. The patient then wears a removable prefabricated
wrist splint for comfort only. [One of us (MNH) instructs the patient
to wear the splint full time.] The wrist is put through gentle
range-of-motion excercises several times a day, independently of finger
motion. Continue the splint for 2–3 weeks for patient comfort only,
during which time encourage progressive use of the hand. Then
discontinue daytime splinting. Continue nighttime wear for an
additional 3–4 weeks if desired (96).
Use physiotherapy selectively for patients who are progressing poorly.
Recovery takes months, so patients who are told that the carpal tunnel
release is a minor operation and full recovery can be anticipated in 2
weeks are grossly misled and usually unhappy.
have shown that grip strength returns to preoperative levels 3 months
after surgery, and pinch strength returns at 6 weeks. Their work
provides us with an indication of when patients ought to be able to
return to their previous levels of occupational activity. Many studies
employ “return to work” as a measure of outcome without clearly
delineating what criteria are to be met to determine when a patient
should return to work. Many factors influence the actual return-to-work
time. Palmar tenderness, a particularly important consideration in
manual laborers, may persist for longer than 6 months. Full recovery of
nerve function may not occur, depending on the severity of nerve
damage. Persistent palmar pain and early recurrent symptoms in workers’
compensation cases are increasing, unsolved problems for patients,
physicians, and industry. Without job modification for these patients,
carpal tunnel release alone may lead to failure in returning the
patient to gainful employment.
factors. Conditions associated with peripheral neuropathies must be
suspected, recognized, and treated before surgery, or concurrently with
the surgical release. Upton and McComas (137)
proposed a “double crush” syndrome as a hypothesis to explain the
failure of distal decompressions in nerves subject to compression in
multiple sites. Many patients with CTS have a cervical radiculopathy.
Others may have concomitant compression at the carpal tunnel as well as
under the PT. Release of the carpal tunnel in these cases may not
relieve symptoms because the more proximal compression remains.
Hypertrophic synovium, if not removed at the time of surgery, may cause
persistent compression despite ligament release.
placement of the incision (i.e., usually too far radially), which
jeopardizes the median nerve and its motor branch, as well as leading
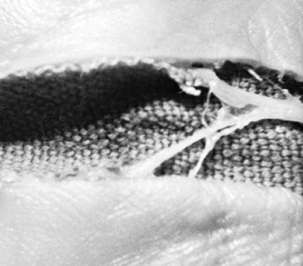
to injury or entrapment of the palmar cutaneous nerve or its branches (Fig. 52.17).
Poor exposure may result in incomplete division of the TCL or in injury
to the superficial palmar arch. Do not use transverse incisions at the
wrist crease for blind decompression of the carpal tunnel.
 |
|
Figure 52.17. The palmar cutaneous branch of the median nerve and its divisions crossing a carpal tunnel incision placed too far radially.
|
to the little finger to sublux during strong gripping. This is often
only a transitory problem. Bowstringing of the flexor tendons with
flexion of the wrist has been noted. This complication is easily
prevented by postoperatively immobilizing the wrist in slight
dorsiflexion for 2–3 days (138), which additionally may prevent adherence of the nerve and tendons to palmar structures and improve grip strength (88).
Reserve reconstruction of the TCL for instances where immobilization of
the wrist in flexion is necessary after carpal tunnel release.
Complications from deficient postoperative management usually result
from swelling and edema or poor control of pain, leading to loss of
finger or wrist motion (particularly if a synovectomy was performed)
and to reflex sympathetic dystrophy. The benefits of a good
postoperative dressing, an aggressive exercise program, and elevation
cannot be emphasized enough.
finding in patients. It varies greatly from case to case in both
severity and duration of its symptoms. Its etiology is poorly
understood, although many theories have been proposed. It generally
subsides and resolves over time. Seradge and Seradge (113)
noted ulnar-sided wrist pain in 1% of their patients after carpal
tunnel release. They attributed this pain to the pisotriquetral joint,
whose mechanics are altered by division of the TCL. Treatment consisted
of excision of the pisiform in cases with transient response to steroid
injection of the joint. We have no evidence to substantiate this theory
or to recommend this approach.
was initially coined to describe compression of the median nerve in the
proximal forearm beneath the pronator teres muscle. Since then, common
usage has evolved so that it now denotes compression of the median
nerve in the proximal forearm and about the elbow (59).
and medial to the biceps muscle in the midarm. In the distal arm, it
crosses the brachial artery to lie medial to it, coursing on the
brachialis muscle. The supracondylar process is an anomalous spur
arising on the anteromedial aspect of the distal humerus, 5 cm proximal
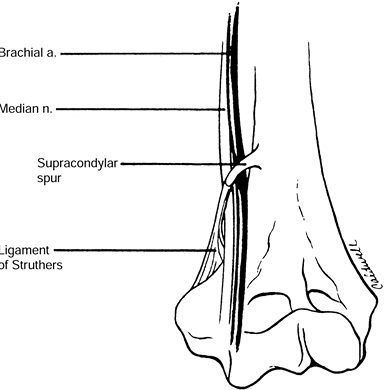
to the medial epicondyle, in as many as 3% of individuals. The ligament
of Struthers is a fibrous band that may arise from the supracondylar
process (spur) of the humerus (7) and attaches
to the medial epicondyle, forming a fibro-osseous tunnel through which
the median nerve and, at times, the brachial artery pass (Fig. 52.18) (66).
The median nerve enters the antecubital fossa coursing beneath the
lacertus fibrosus (bicipital aponeurosis), then travels between the
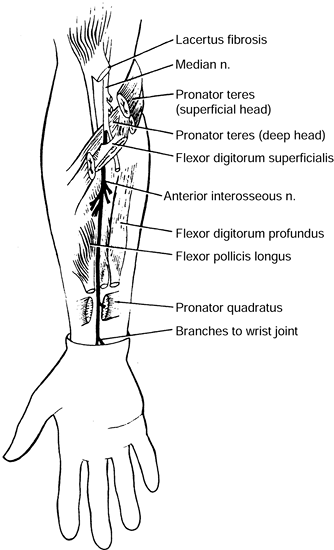
superficial (humeral) and deep (ulnar) heads of the PT muscle. It
continues distally beneath the arch of origin of the flexor digitorum
superficialis (FDS), coming to lie between it and the flexor digitorum
profundus (FDP). The median nerve maintains this relationship
throughout its course to the wrist. Potential sites of compression
include the supracondylar process and the ligament of Struthers, the
lacertus fibrosus, the arch of the origin of the PT, and the arch of
the origin of the FDS (Fig. 52.26, Table 52.4).
 |
|
Figure 52.18. The ligament of Struthers. (From Stern PJ, Fassler PR. Pronator Syndrome. In: Gelberman RH, ed. Operative Nerve Repair and Reconstruction. Philadelphia: Lippincott, 1991:995, with permission.)
|
 |
|
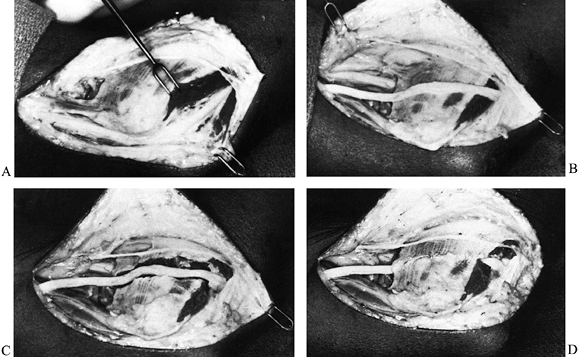
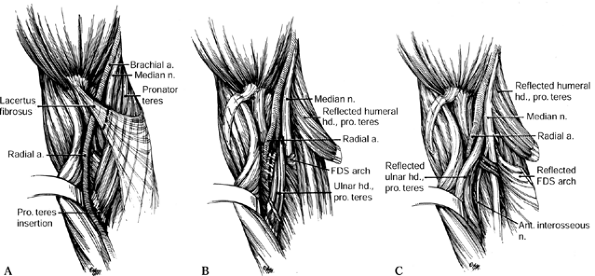
Figure 52.26. A: Division of the lacertus fibrosus. B: Exposure of the median and anterior interosseous nerves and the arch of the superficialis. C:
The radial origin of the superficialis muscle elevated by subperiosteal dissection to expose the deep volar compartment and the AIN. (From Eversmann WW Jr. Entrapment and Compression Neuropathies. In: Green DP, ed. Operative Hand Surgery. New York: Churchill Livingstone, 1982:1341, with permission.) |
 |
|
Table 52.4. Potential Compression Sites of the Median Nerve in Pronator Syndrome
|
nerve at this level, including numbness in the radial three and
one-half digits and thenar weakness, may be attributed to CTS. In
pronator syndrome, unlike in CTS, paresthesias are typically absent at
night. Important additional symptoms that may help differentiate this
condition from CTS are pain in the anterior aspect of the proximal
forearm and numbness in the thenar region—the territory of the palmar
branch of the median nerve that does not travel through the carpal
tunnel, having branched from the median nerve several centimeters
proximal to the wrist. Sensory threshold testing detects decreased
sensation in the radial three and one-half digits, as in CTS, with the
addition of numbness in the thenar eminence, the area innervated by the
palmar cutaneous branch (Fig. 52.8A). The findings of weakness and atrophy in the thenar musculature
may be indistinguishable from those seen in CTS, although they are less severe and are most often absent.
further differentiate pronator syndrome from CTS is provocative
testing. Phalen’s test should be negative, but it may be positive (47,95).
Tinel’s percussion test, in which the median nerve is percussed at the
level of the pronator muscle, elicits paresthesias in the distribution
of the median nerve in the proximal forearm rather than at the carpal
tunnel (Fig. 52.19). To perform the pronator
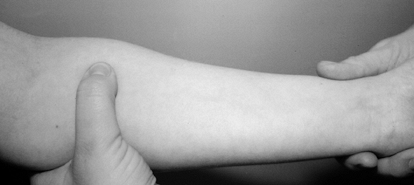
compression test, place direct thumb pressure just proximal and lateral
to the proximal edge of the PT muscle belly (Fig. 52.20).
(The brachial pulse will be palpable lateral to the nerve.) A positive
test is reproduction of paresthesias in the median-nerve-innervated
digits within 30 seconds, and it supports the diagnosis of pronator
syndrome (38). According to Olehnik et al. (95),
the pronator compression test is the most accurate diagnostic test.
Several tests have been designed to give clues to the specific site of
compression of the median nerve in pronator syndrome. The production of
pain or aggravation of paresthesias with simultaneous resisted forearm
supination and resisted elbow flexion beyond 120° indicates probable
entrapment of the median nerve at the lacertus fibrosus (Fig. 52.21) (7,33,58,115,122).
Entrapment of the nerve between the two heads of the PT muscle is
indicated by elicitation of paresthesias in the median nerve sensory
distribution with resisted forearm pronation while the elbow is slowly
extended from full flexion (Fig. 52.22) (33,58,121,122).
Paresthesias elicited in the radial three and one-half digits with
resisted independent flexion of the proximal interphalangeal (PIP)
joint of the long finger suggest entrapment beneath the superficialis
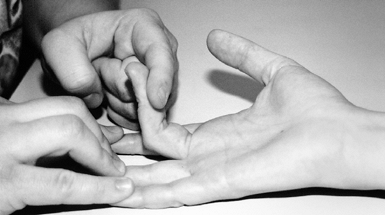
arch of origin (Fig. 52.23, Table 52.5) (33,122).
 |
|
Figure 52.19. Tinel’s percussion test in the proximal forearm for evaluation of pronator syndrome.
|
 |
|
Figure 52.20. The pronator compression test.
|
 |
|
Figure 52.21. Testing for entrapment of the median nerve at the lacertus fibrosus in pronator syndrome.
|
 |
|
Figure 52.22. Testing for entrapment of the median nerve at the pronator in pronator syndrome.
|
 |
|
Figure 52.23. Testing for entrapment of the median nerve at the arch of the flexor digitorum superficialis in pronator syndrome.
|
 |
|
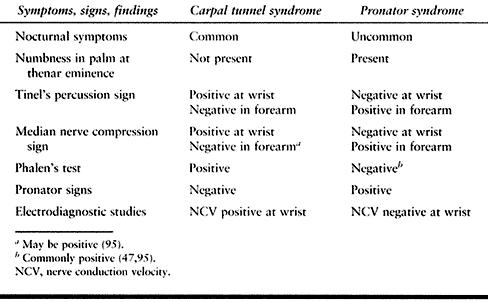
Table 52.5. Carpal Tunnel versus Pronator Syndrome
|
studies and radiographs of the elbow. Median nerve conduction
velocities from the elbow to the wrist are decreased in less than one
third of cases (16,47,95).
The value of obtaining these studies is to evaluate for the presence of
CTS. A normal conduction velocity at the level of the wrist supports
proximal compression of the median nerve in the presence of symptoms
and signs of pronator syndrome. A study consistent with CTS indicates
the necessity
of
treatment directed at the wrist but does not rule out a double crush
phenomenon, with compression both at the carpal tunnel and in the
proximal forearm. Failed carpal tunnel releases that respond to
subsequent operative release in the proximal forearm support the
occurrence of simultaneous compression of the nerve at these two sites (95).
Four views of the elbow—anteroposterior (AP), lateral, and two
obliques—are obtained to evaluate the presence of a supracondylar
process. Although its presence is suggestive of compression of the
median nerve beneath the ligament of Struthers, it is not
pathognomonic; this is a very rare site of entrapment. The absence of a
supracondylar process does not rule out the presence of a ligament of
Struthers and entrapment at this site (Table 52.6).
 |
|
Table 52.6. Pronator Syndrome Summary
|
medications, splinting, and rest for 4–6 weeks. Modification of
activities is particularly important because symptoms are often related
to repetitive elbow flexion and extension and to forearm pronation and
supination. Physiotherapy in the form of massage, stretching, and
iontophoresis to mobilize and relax potentially tight structures may be
helpful. Carefully placed steroid injections in the site of maximal
tenderness and pain (but not into the nerve) may be useful for
diagnostic and therapeutic purposes.
respond to nonoperative care. Surgery is indicated in those cases where
symptoms persist longer than 6 weeks to 3 months following adequate
conservative management.
80% to 90% of cases (38,47,59,95).
Complete relief of symptoms occurs in only about one third of those who
improve. There appears to be no preoperative indicator of which
patients will have the better results (95). As
in CTS, return to work will depend in part on the demands of the
patient’s particular vocation. Of the 36 patients in the series
reported by Olehnik et al. (95), 25 returned to
their preoperative or similar occupations and eight returned to work
but had to change jobs. The final work status of the remaining three
patients was unknown.
whether the site of compression is above or below the elbow. If there
is evidence of compression under an arcade of Struthers, center the
surgical exposure at the radiographic projection of the supracondylar
process. If the compression is below the elbow, extend the incision
distal to the elbow flexion crease onto the forearm to expose the
pronator and the arch of the FDS.
-
Center the incision over the
supracondylar process and make it in line with the medial neurovascular
bundle anterior to the medial intermuscular septum (Fig. 52.24).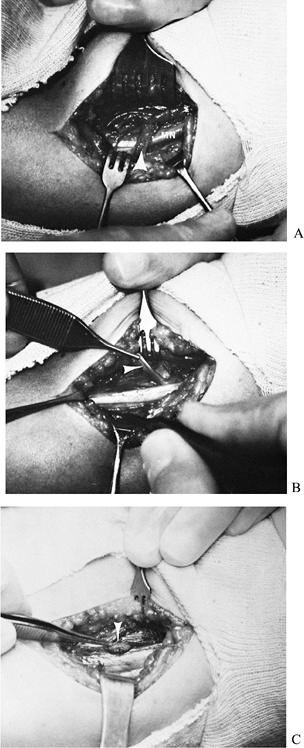 Figure 52.24. Entrapment of the median nerve (MN) under a ligament of Struthers. A: Initial appearance. B: The ligament divided and elevated (arrow). C: Supracondylar process (arrow).
Figure 52.24. Entrapment of the median nerve (MN) under a ligament of Struthers. A: Initial appearance. B: The ligament divided and elevated (arrow). C: Supracondylar process (arrow). -
Palpate the supracondylar process and expose the ligament of Struthers.
-
The median nerve, brachial artery, and
sometimes the ulnar nerve lie within the proximity of the supracondylar
process; therefore, proceed; cautiously with dissection. -
Divide the ligament of Struthers and excise the supracondylar process.
-
Continue distally and divide the lacertus
fibrosus. This is done routinely in decompression of the median nerve
for these cases and for others that do not involve compression at the
supracondylar process. The lacertus fibrosus may act as a compressive
band across the flexor muscle mass in supination and hence should be
divided with any exploration of the median nerve. -
If you do not find a ligament of Struthers, you must discover the area of compression by additional dissection distally.
-
Begin the incision along the projection of the medial neurovascular bundle, 5 cm proximal to the elbow flexion crease.
-
Continue it in a gentle curve along the elbow flexure, and extend it distally past the superficialis arch (Fig. 52.25).
![]() Figure 52.25. Extensile approach for the exposure of the median nerve at the elbow and proximal forearm.
Figure 52.25. Extensile approach for the exposure of the median nerve at the elbow and proximal forearm. -
Protect the medial antebrachial cutaneous nerve from injury.
-
Raise full-thickness flaps radially and ulnarly off the forearm muscle fascia.
-
Identify the median nerve proximal to the lacertus fibrosus and the PT (Fig. 52.26A).
-
The lacertus fibrosus originates from the
anteromedial aspect of the musculotendinous junction of the biceps and
travels distally and medially, crossing the median nerve and brachial
artery to blend into the fascia of the flexor pronator muscle mass.
Divide it in line with the course of the median nerve. -
Follow the median nerve distally as it passes between the two heads of the PT.
-
Detach the superficial head of the
pronator from its distal conjoined tendon with the deep head, using a
stepcut or long oblique incision designed to allow reattachment of the
superficial head with relative lengthening (122). This requires dissection distal to the midforearm level. -
Reflect the superficial head ulnarly (Fig. 52.26B).
-
Identify the arch of the FDS, which lies
in the distal portion of the wound, deep to the pronator. Expose the
FDS by reflection of the superficial head of the pronator. -
Follow the median nerve as it courses deep to the arch of the FDS.
-
Detach the radial origin of the FDS (Fig. 52.26C)
or, alternatively, divide it in line with its fibers between its radial
and ulnar halves to expose and decompress the median nerve. -
Reattach the superficial pronator at a point proximal to its original insertion or in a lengthened position, using the stepcut.
-
Close the skin.
The fascia was then split longitudinally to allow adequate access to
the soft-tissue structures in the antecubital fossa to the midforearm.
Deep digital palpation and dissection were used to evaluate for
compression of the nerve proximally by a ligament of Struthers. They
additionally released intramuscular fascial bands of the PT and FDS
that crossed the nerve. The authors of this study point out the
importance of tracing the nerve along its course, releasing all fibrous
bands and vascular structures that are potential sites of compression.
 |
|
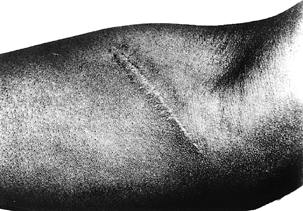
Figure 52.27.
An oblique incision 3 months after surgery for decompression of the median nerve in pronator syndrome. (From Olehnik WK, Manske PR, Szerzinski J. Median Nerve Compression in the Proximal Forearm. J Hand Surg [Am] 1994;19:121, with permission.) |
dressing and a posterior plaster splint with the elbow flexed at right
angles and the forearm in semipronation. Encourage shoulder and finger
motion immediately after the operation. After 5 days, discontinue
immobilization; allow the patient to resume elbow flexion and extension
and forearm rotation and to gradually return to full activities as
tolerated.
responsible for most complications. The most important is failure to
release constriction at one of the four sites of possible entrapment.
You must also address all other potential sources of compression by
visualizing the nerve along its course. Avoid accidental damage to
branches of the median nerve by initially exposing and dissecting the
nerve along its lateral aspect, which is free of branches.
median nerve that is essentially entirely motor except for a few
terminal branches that are sensory to a portion of the carpus. The most
common pattern of muscles innervated by the AIN includes the FDP to the
index finger, the flexor pollicis longus (FPL), and the pronator
quadratus (PQ). This innervation pattern varies significantly, which
can cause confusion during clinical examination. The nerve arises from
the dorsal aspect of the median nerve as it passes between the two
heads of the PT. In some cases, it passes deep to the deep head of the
PT. It passes beneath the arcade of the FDS and courses distally along
the anterior surface of the interosseous membrane between the FDP and
the FPL, accompanied by the anterior interosseous artery. The AIN gives
branches to the FPL and the radial aspect of the FDP muscles
approximately 4 cm distal to its origin from the median nerve (Fig. 52.28).
Compression may be caused by one of many structures, including the deep
head of the PT, the FDS, accessory muscles (e.g., Gantzer’s muscle, and
an accessory FPL) (28,74), aberrant vessels (e.g., anomalous radial artery), and tendinous bands along the course of the nerve.
 |
|
Figure 52.28.
The course and innervations of the anterior interosseous nerve. (From Chidgey LK, Szabo RM. Anterior Interosseous Nerve Palsy. In: Szabo RM, ed. Nerve Compression Syndromes: Diagnosis and Treatment. Thorofare, NJ: Slack, 1989:153, with permission.) |
proximal forearm and sometimes in the wrist. This occurs at rest and is
exacerbated by activities. There is no sensory deficit with AIN
syndrome, which is different from carpal tunnel and pronator syndromes.
Patients may note difficulty with activities such as writing, or
weakness in tip pinch. Frequently, there is a history of a single
episode of strong contraction of elbow, wrist, and finger flexors
accompanied by pain and followed shortly thereafter by motor loss.
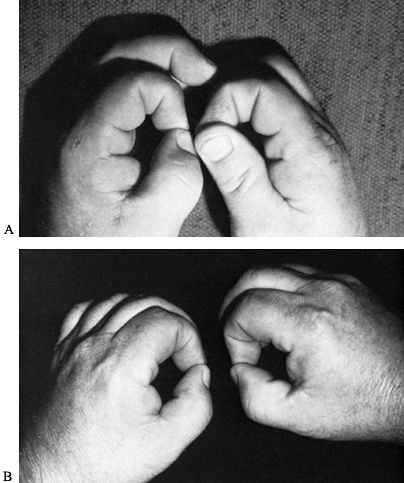
Weakness of the FPL and of the radial half of the FDP makes it
difficult or impossible for the patient to tip pinch with the index
finger and thumb (i.e., to make the so-called OK sign). The lack of
long flexors results in a hyperextension attitude of the distal joints
of these two digits (Fig. 52.29) (119).
You can test the weakness of the PQ by asking the patient to pronate
against resistance, with the elbow flexed to neutralize the stronger
PT. However, isolated paralysis of any one or a combination of these
muscles has been reported (50).
 |
|
Figure 52.29. Incomplete anterior interosseous nerve syndrome. A: Preoperative loss of flexion of the right thumb. B: Postoperative result.
|
rupture and AIN syndrome in the patient with acute presentation. This
is done best by looking for the tenodesis effect of the FPL and index
FDP (79). Innervation anomalies add variability
to the classic examination findings. Martin-Gruber connection (median
to ulnar motor nerve connection in the forearm) occurs in 15% of
individuals; 50% of these arise from the AIN. The presence of this
anomaly may cause weakness of additional intrinsic muscles
of
the hand in patients with AIN syndrome. Additionally, in 50% of
individuals, the FDP to the index is innervated by branches from the
median nerve, not the AIN (50).
The diagnosis is usually confirmed by EMG studies. Nerve conduction
studies are usually not affected, although side-to-side differences may
be noted (Table 52.7).
 |
|
Table 52.7. Anterior Interosseous Nerve Syndrome Summary
|
neurodiagnostic studies 2–3 weeks after injury, and again at 6 and 12
weeks if no clinical improvement is noted. Chronic cases, presenting
after 6 weeks, should have neurodiagnostic examination performed
initially and the patient should be followed clinically; if no
improvement is noted, perform another study at 12 weeks after the
injury. Although no specific protocols have been evaluated,
nonoperative treatment may consist of avoidance of exacerbating
activities, immobilization of the elbow in flexion and the forearm in
pronation, and the use of NSAIDs.
spontaneous recovery, although in some instances this has taken up to 2
years. Most authors agree that recovery can be enhanced by surgical
exploration when spontaneous recovery is not apparent or is slow (50).
Recovery is generally complete within 6 months after surgery.
Persistence of the motor symptoms without signs of significant
improvement for 8–12 weeks is an indication for surgical exploration
and decompression (122). Evidence of clinical motor recovery or signs of reinnervation on EMG would call for further observation.
-
Use the same incision as for pronator syndrome.
-
Divide the lacertus fibrosus to allow access to the median nerve as it passes beneath the superficial head of the PT (Fig. 52.26A).
-
The AIN branches from the median nerve
just distal to the proximal border of the superficial head of the
pronator. Trace it beneath this muscle. This is a common site of
entrapment. -
If necessary for exposure, you may detach the superficial head of the pronator (Fig. 52.26B).
-
Trace the nerve distally as it passes
beneath the FDS. If necessary, detach the origin of this muscle or
divide its fibrous arch, as in pronator syndrome exploration, to trace
the nerve distally (Fig. 52.26C). -
Trace the nerve as it travels along the anterior interosseous membrane between the FPL and FDP muscles.
-
Terminate the distal dissection when you visualize the branches to the deep flexors.
-
Reattach the FDS muscle. (This may be done posterior to the median nerve if you feel that anterior transposition is necessary.)
-
Reattach the PT superficial head deep to the median nerve and AIN.
-
Close the skin and place a long-arm splint.
the entire nerve and divide all suspected offending structures. The
postoperative management is essentially identical to that after
treatment of the pronator syndrome.
for the carpal tunnel and PT syndromes. The main problem is an error in
diagnosis, particularly for the patient with an incomplete syndrome
involving either the thumb or the index long flexors, but not both (Fig. 52.29) (50). Such a
presentation may lead to the erroneous diagnosis of tendon rupture and
to a negative tendon exploration. Perform the tenodesis test with the
wrist in maximal extension along with MP joint and PIP joint extension.
This should produce slight flexion at the distal interphalangeal (DIP)
joint of the index and the IP joint of the thumb with AIN syndrome but
not with tendon rupture (79).
cases with an acute onset of pain in the forearm followed by weakness
in the muscles normally affected in AIN syndrome a few days to weeks
later (at times associated with a febrile illness, vaccination, or
unrelated surgery), especially in bilateral cases (149).
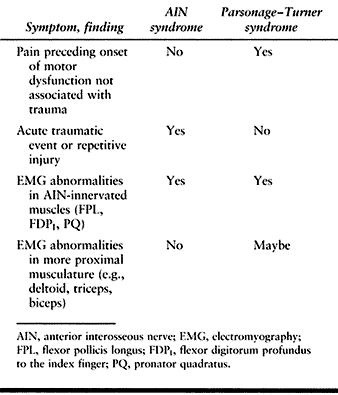
In Parsonage–Turner syndrome, there will also be shoulder pain and
involvement of the shoulder muscles at times. AIN compression syndrome
generally presents after an acute injury or in conjunction with
repetitive activity, whereas brachial neuritis is insidious in nature.
In Parsonage–Turner syndrome there is pain not associated with an
injury, which may involve more proximal areas of the upper extremity
than that seen in AIN syndrome. Parsonage–Turner syndrome often
produces EMG results similar to those of AIN syndrome in the FPL, FDP,
and PQ. Sampling of more proximal muscles innervated by the brachial
plexus, such as the deltoid, may also demonstrate EMG abnormalities,
which is different from AIN entrapment (Table 52.8).
 |
|
Table 52.8. AIN Syndrome versus Parsonage–Turner Syndrome
|
surgically or nonoperatively. Surgical exploration and decompression
are reserved for cases associated with trauma.
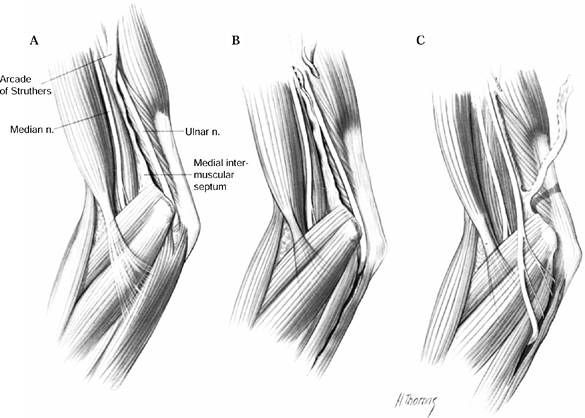
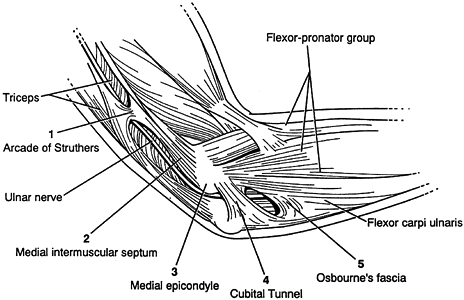
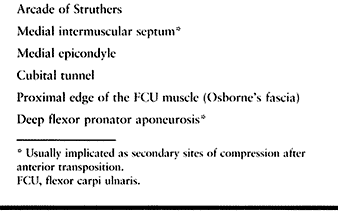
nerve is about the elbow; this condition is known as cubital tunnel
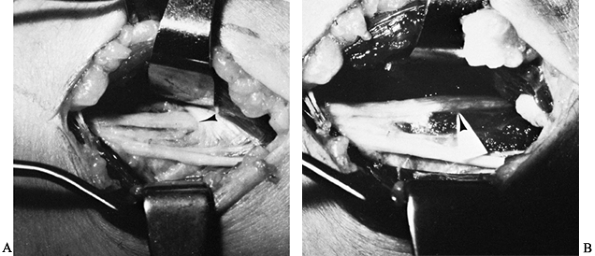
syndrome. There are five potential compression sites of the nerve that
occur along its course. At the midarm level, the ulnar nerve pierces
the medial intermuscular
septum
and runs distally posterior to it. Approximately 8 cm proximal to the
medial epicondyle, the nerve may pass through an inconstant fibrous
tunnel, the arcade of Struthers, formed by a band connecting the medial
intermuscular septum to the tendon of the medial head of the triceps.
This is the first potential site of compression (Fig. 52.30).
The nerve continues posterior to the intermuscular septum. The edge of
the septum becomes a second potential site of compression, usually
after failed anterior transposition. The nerve then comes to lie in the
retrocondylar groove of the medial epicondyle, the third potential site
of compression, where it gives off a few articular sensory branches to
the elbow joint; it then enters the cubital tunnel, the fourth
potential site of compression. The cubital tunnel is a fibro-osseous
canal formed by the medial epicondyle anteriorly, the ulnohumeral
ligament posterolaterally, and a structure termed the cubital tunnel
retinaculum (92), which is superficial and
forms the roof of the tunnel. The fifth potential site of compression
occurs as the nerve passes under Osborne’s fascia, a thick fascial
layer that connects the heads of the FCU to the medial epicondyle and
olecranon in nearly 80% of individuals and is often confluent with the
cubital tunnel retinaculum (24). The nerve then
courses to enter the forearm between the two heads of the FCU. At this
level, it gives off several motor branches to the FCU. It comes to lie
medial to the FDP, piercing the deep flexor pronator aponeurosis—a
fascial structure serving as a common origin and aponeurosis of the
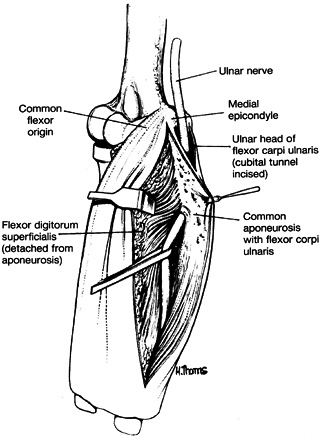
humeral head of the FDS and the FCU—as it exits the cubital tunnel (61).
This aponeurosis is the sixth and most distal potential compression
site, and it, like the medial intermuscular septum, is usually a site
of secondary compression after anterior transposition (Fig. 52.31, Table 52.9).
 |
|
Figure 52.30. The first five potential compression sites of the ulnar nerve in cubital tunnel syndrome: (a) arcade of Struthers, (b) medial intermuscular septum (considered a secondary site of compression), (c) medial epicondyle, (d) cubital tunnel, (e)
Osborne’s fascia. (From Osterman AL, Davis CA. Subcutaneous Transposition of the Ulnar Nerve for Treatment of Cubital Tunnel Syndrome. Hand Clin 1996;12:421, with permission.) |
 |
|
Figure 52.31. The sixth potential site of compression of the ulnar nerve in cubital tunnel syndrome, the flexor pronator aponeurosis.
|
 |
|
Table 52.9. Potential Compression Sites of the Ulnar Nerve in Cubital Tunnel Syndrome
|
Both constriction of the nerve and traction are implicated in its
dysfunction. Flexion of the elbow has been shown to cause increased
intraneural pressure in the ulnar nerve at the cubital tunnel and
decreased volume in the cubital tunnel itself (92). The ulnar nerve at the elbow normally
glides to accommodate elbow flexion (6). When tethered by scar or fibrosis, the ulnar nerve experiences traction forces, which affect nerve function.
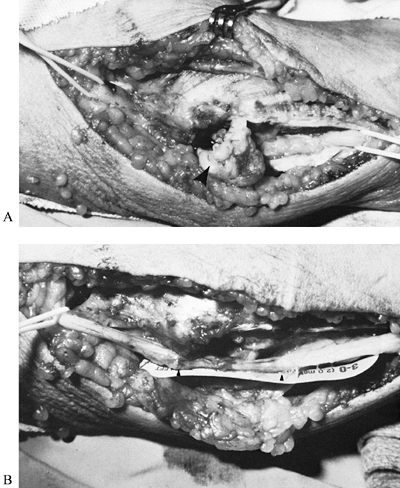
deformity (e.g., cubitus valgus), malunion or nonunion of the medial
epicondyle, elbow instability, spurs or bone fragments within the floor
of the cubital tunnel (Fig. 52.32), tumors,
abnormal muscles (e.g., anconeus epitrochlearis), and a nerve that
subluxates or dislocates repeatedly over the medial epicondyle.
 |
|
Figure 52.32. A: Compression of the ulnar nerve at the elbow by a large osteochondral body (arrow). B: Severe nerve constriction (between arrows) after removal of the osteochondral fragment.
|
paresthesias along the ulnar nerve distribution, the small finger, the
ulnar half of the ring finger, and the ulnar aspect of the hand (Fig. 52.8).
This is exacerbated by leaning on the elbow or positioning it in
flexion. Aching pain may be referred to the medial aspect of the elbow.
Numbness on the medial half of the forearm is not usually present.
Weakness of grip or loss of dexterity in the fingers may accompany these symptoms.
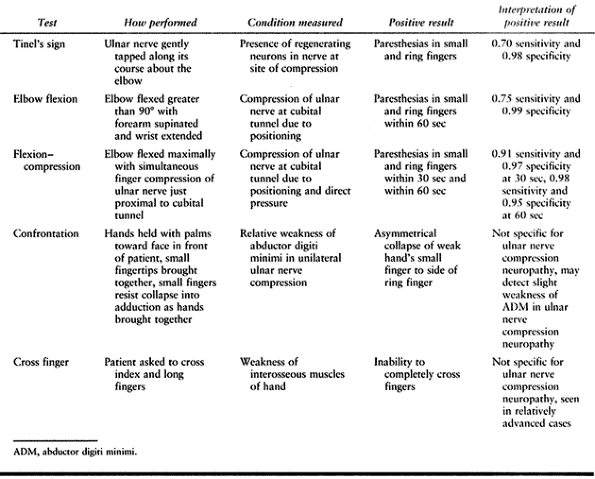
provocative testing, and motor examination. Threshold sensory
evaluation, including Semmes–Weinstein monofilaments and vibratory
testing, is most sensitive and is recommended. Two-point discrimination
yields abnormal results in more advanced cases. Sensation is decreased
in the ring and small fingers as well as in the ulnar half of the
dorsum of the hand, which indicates compression proximal to the origin
of the dorsal cutaneous branch of the ulnar nerve and therefore
proximal to the wrist.
compression, as well as uncover dynamic forms of the disorder. Tinel’s
test is positive if paresthesias are elicited in the distribution of
the nerve when it is gently percussed. The location of percussion with
maximal elicitation of symptoms may indicate the site of compression.
Tinel’s test is sensitive but not specific, giving a high rate of false
positive results at the cubital tunnel (23). The elbow flexion test described by Buehler and Thayer (17) and others (101),
which is flexion of the elbow beyond 90° with the forearm in supination
and the wrist in extension, is positive when symptoms are produced
within 1 minute, and it may localize compression to the level of the
elbow. Novak et al. (90) modified this test to
include direct compression of the ulnar nerve with the examiner’s
finger just proximal to the cubital tunnel while the elbow is flexed
maximally (Fig. 52.33). The test is considered
positive when paresthesia symptoms are produced in the ulnar nerve
distribution within 30 seconds. This test is reported to have 0.91
sensitivity and 0.97 specificity. Evaluate potential subluxation of the
ulnar nerve over the medial epicondyle by direct palpation while the
elbow is brought from full extension to full flexion. Ulnar nerve
subluxation is seen in 16% of normal individuals (20)
and therefore is not considered pathologic per se. If associated with
neuritic symptoms, it may be the cause of injury to the nerve.
Palpation may also elicit tenderness and disclose an enlarged,
sensitive nerve.
 |
|
Figure 52.33. The flexion compression test for cubital tunnel syndrome.
|
advanced disease. Atrophy may be seen most commonly in the hypothenar
musculature and in the first dorsal interosseous muscle. When weakness
is present, it involves not only the ulnar innervated intrinsic muscles
but typically the FDP to the ring and small fingers. When both ulnar
innervated intrinsic and extrinsic muscles are involved, the ulnar claw
hand, a sign of intrinsic–extrinsic imbalance, does not appear as
severe. Grip strength testing is often normal unless there is
significant loss of power to the long flexors of the ring and small
finger. Pinch strength, especially key position, is decreased as a
result of loss of strength to the flexor pollicis brevis (FPB), the
adductor pollicis, and the first dorsal interosseous muscle. Froment’s
sign (hyperflexion of the IP joint) and Jeanne’s sign (hyperextension
of the MP joint of the thumb) are elicited during key pinch (Fig. 52.34).
Weakness or paralysis of the FPB concentrates the flexion force of the
FPL at the IP joint, causing hyperflexion of this joint (11). The EPL compensates for the adductor pollicis and enhances hyperextension
of the MP joint of the thumb because it is unopposed by the intrinsic flexor of the thumb (the EPB).
 |
|
Figure 52.34. Froment’s sign and Jeanne’s sign, seen with intrinsic muscle weakness in ulnar nerve palsy.
|
the first dorsal interosseous muscle against resistance, and measure it
by side-to-side comparison in unilateral cases. In some individuals,
contribution of innervation to this muscle from the median nerve can
occur (109), and therefore this test is not
specific. Evaluation of the abductor digiti quinti is also subjective.
Side-to-side comparison is facilitated by a confrontation test. Ask the
patient to maximally abduct her fingers while holding her hands out in
front, palms facing her. She brings the tips of her small fingers
together and, while she resists collapse of the abducted small fingers,
she brings her hands together. In unilateral cases with abductor
weakness, the weak hand’s small finger collapses to the side of the
ring finger (Fig. 52.35).
 |
|
Figure 52.35. The confrontation test.
|
extension of the fingers at the MP joint level. It indicates
interosseous dysfunction. The cross finger test (30)
is very useful and also evaluates function of the interossei. Ask the
patient to cross his index and long fingers. Patients with ulnar nerve
dysfunction often have trouble performing this task. Atrophy and
weakness of intrinsic muscles innervated by the ulnar nerve indicate a
moderate to severe lesion of the nerve (Table 52.10).
 |
|
Table 52.10. Provocative Tests for Cubital Tunnel Syndrome
|
deformity, and suspected arthritis, and where there is incomplete range
of motion. AP, lateral, and oblique views are obtained, as well as
axial views of the distal humerus and olecranon for evaluation of the
ulnar groove.
These are helpful in confirming the diagnosis and in classifying the
case for treatment and prognostic purposes. NCV evaluations of the
segment of the ulnar nerve across the elbow are considered significant
if conduction values are reduced by 33% (33).
Segmental “inching” studies may be helpful in localizing the site of
compression, especially in previously operated cases with recurrence of
symptoms. EMG evaluation of the ulnar innervated muscles can provide
information on chronicity and progression as well as severity, and it
may indicate the approximate site of compression. More importantly, EMG
needle examination coupled with somatosensory evoked potential (SSEP)
evaluates for radiculopathies and more proximal lesions, including
thoracic outlet syndrome (TOS), which is suspected especially in cases
of bilateral ulnar nerve symptoms (Table 52.11).
 |
|
Table 52.11. Cubital Tunnel Syndrome Summary
|
He classified patients into mild, moderate, and severe categories
depending on physical and electrodiagnostic findings. Surgical
treatment options and the relative results with respect to the patient
classification will be discussed.
 |
|
Table 52.12. Staging of Ulnar Nerve Compression at the Elbow
|
to have an effect on cubital tunnel syndrome. Injections are advocated
by some. Two of us (MNH and JT) occasionally use them. Water-soluble
steroid is injected at the proximal aspect of the cubital tunnel, not
into the tunnel. Take great care to avoid injury to the ulnar nerve,
which is at risk. Most agree, though, that injection is to be avoided
because of the great risk of injury to the nerve, especially if
injection is made into the cubital tunnel where the nerve is tightly
confined.
tunnel syndrome includes patient education and splinting. The
occupational or physical therapist instructs the patient in nerve
protection. This includes avoidance of repetitive flexion and extension
of the elbow, of prolonged positioning of the elbow in the flexed
position, and of leaning on the medial aspect of the elbow. Adjustments
are made in the height of work stations and seats when indicated, so
that the elbow is flexed no more than 30° during work. Elbow pads may
be worn to prevent direct pressure on
the nerve in its subcutaneous position (Fig. 52.36).
Hand therapists can provide Thermoplast or pillow extension splints
that hold the elbow in 30° to 45° of flexion during sleep to prevent
hyperflexion (Fig. 52.37). Recheck the patient
after 3 months of treatment. In mild cases and some moderate ones,
patients who comply with these instructions may have a good response
and be able to avoid surgery.
 |
|
Figure 52.36. Commercially available elbow pads worn to protect the ulnar nerve from mechanical irritation.
|
 |
|
Figure 52.37. Thermoplast night splints custom fabricated by a hand therapist.
|
nonoperative care is an indication for surgery. Inability of the
patient to comply with nonsurgical care in mild or moderate cases is a
relative indication. Severe involvement, including motor dysfunction,
requires surgical intervention. Ulnar neuropathies associated with
deformity, bony lesions, tumors, and elbow instabilities, among others,
require attention to the pathology contributing to the nerve
compression as well as the nerve itself.
condition of the patient all influence the outcome of treatment.
Patients with intermittent symptoms, no atrophy, and mild
electrodiagnostic findings respond well to nonoperative treatment (32).
The choice of surgical procedure in some instances may also have an
effect. In most uncomplicated cases, return of sensation and motor
function occurs within 6 months. Nouhan and Kleinert (89)
evaluated 33 limbs that had been followed for an average of 49 months.
Preoperative nerve compression was classified as mild in 6, moderate in
7, and severe in 20. Surgical treatment consisted of submuscular
transposition with Z-lengthening of the flexor pronator mass. There were 97%
good to excellent results overall; the preoperative classification did not influence the final result.
evaluated the results of 160 cases undergoing cubital tunnel release
and medial epicondylectomy. Preoperative severity of compression was
mild in 7%, moderate in 86%, and severe in 7%. There were 87% good, 12%
fair, and 1% poor results (failure of treatment). Factors that
increased the rates of symptom recurrence in their patients included
female sex, the presence of concomitant CTS or TOS in the same
extremity, age in the third or fourth decade, and patients who did not
return to work within 3 months after surgery. Limb dominance, length of
preoperative conservative care, and EMG results did not have a
relationship to recurrence rate.
patients in the mild group had excellent results from surgery no matter
what technique was used. Patients in the moderate group did not improve
without surgery, and decompression in situ
was not effective. Medial epicondylectomy produced 50% excellent
results with a high recurrence rate, whereas submuscular transposition
gave 80% excellent results with the lowest recurrence rate. In severe
patients, surgery resulted in less than 50% excellent results for
sensory recovery and up to 25% for motor recovery. Recurrence developed
in 30% of these patients. In this group, the poorest results were in
those undergoing intramuscular transposition, and the best were in
those receiving submuscular transposition and internal neurolysis.
of the ulnar nerve, consider additional diagnostic studies to further
delineate the pathology. Computed tomography (CT) scans can further
characterize suspected bony abnormalities, including space-occupying
spicules or fragments, fracture callus, and malunions or nonunions.
Suspected tumors are best visualized by magnetic resonance imaging
(MRI) evaluation. Progressive deformities (e.g., cubitus valgus due to
nonunion of the lateral condyle) must be stabilized at the time of
decompression and transposition of the nerve, or in a staged fashion.
Patients with previously failed decompressions should undergo inching
nerve conduction velocities to better localize the site of compression,
most often in the most proximal or distal portion of the previous
surgical exposure.
any of the commonly employed operative methods. The choice of operative
technique is greatly influenced by the cause of the compression, the
patient’s age, and systemic conditions such as diabetes or alcoholism.
Operative techniques include simple decompression, medial
epicondylectomy, subcutaneous transposition, intramuscular
transposition, and submuscular transposition. The nerve may be
decompressed by simple division of the arch of origin of the FCU (or
the cubital tunnel) in the following cases: (a) if clinical findings
(e.g., a localized nerve percussion sign) suggest isolated entrapment
of the nerve beneath the arch (or cubital tunnel retinaculum), (b) if
the nerve presents a constricting groove caused by this arch (or
retinaculum), as visualized during operation (Fig. 52.38),
or (c) if the nerve is vulnerable to manipulation (e.g., in older
patients or patients with diabetic or alcoholic neuropathy) (148).
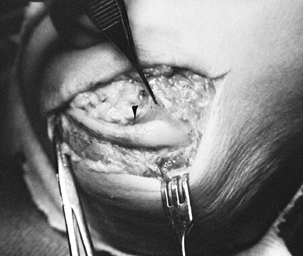 |
|
Figure 52.38. Well-defined constriction of the ulnar nerve (arrow), seen after division of the arch of origin of the flexor carpi ulnaris.
|
neuropathies associated with elbow deformity, abnormalities of the
cubital canal, and the subluxing or dislocating ulnar nerve,
particularly in the younger population and in the more severe cases
with evidence of motor involvement (66,69).
A particular indication for medial epicondylectomy is the ulnar
neuropathy associated with nonunion of a fracture of the medial
epicondyle. Routine use of this technique has been shown to be
effective (48,60). Some prefer anterior subcutaneous transposition over submuscular transposition (1,31,99,104).
Subcutaneous transposition is commonly employed as part of other
operative procedures about the elbow, including fracture reduction,
elbow arthroplasty, and neurorrhaphy of the ulnar nerve in cases where
there is loss of nerve length.
patience and diligence to prevent damage to the nerve, its branches,
and its accompanying blood supply. This is aided by use of
magnification, fine instruments, and bipolar cautery. A trained
surgical assistant is invaluable in facilitating these procedures and
in adding a level of safety to prevent potential complications related
to nerve injury.
-
Start the skin incision equidistant
between the olecranon and the medial epicondyle; extend it 3–4 cm
proximally and 6–8 cm distally (Fig. 52.39A).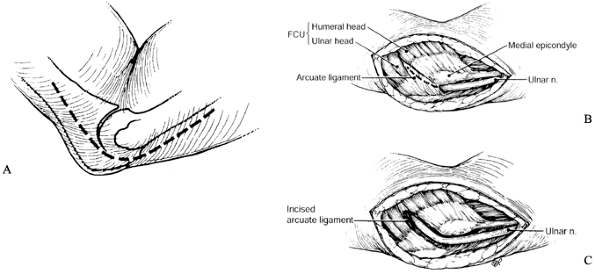 Figure 52.39. In situ decompression of the ulnar nerve at the cubital tunnel. A: Skin incision. B: Division of the arcuate ligament (cubital tunnel retinaculum). C:
Figure 52.39. In situ decompression of the ulnar nerve at the cubital tunnel. A: Skin incision. B: Division of the arcuate ligament (cubital tunnel retinaculum). C:
Extension of the incision into the interval between the two heads of
the flexor carpi ulnaris to ensure distal decompression. (From Ferlic
DC. In Situ Decompression of the Ulnar Nerve at the Elbow. In: Gelberman RH, ed. Operative Nerve Repair and Reconstruction. Philadelphia: Lippincott, 1991;1063, with permission.) -
Isolate the ulnar nerve before it enters the cubital canal.
-
Delineate the arch of origin of the FCU,
elevate it from the nerve, and divide both it and Osborne’s fascia
while protecting the nerve under direct visualization (Fig. 52.39B). -
Extend this division distally between the two heads of the FCU to allow room for the nerve (Fig. 52.39C).
-
Flex and extend the elbow, and make sure
the nerve does not sublux. If subluxation occurs, be prepared to
transpose the nerve or perform a medial epicondylectomy. -
Release the tourniquet for careful hemostasis.
-
Do not close the FCU heads beneath the ulnar nerve.
-
Close only the skin.
-
Apply a well-padded dressing.
immediate active flexion and extension of the elbow. Subluxation of the
nerve after decompression is rare. On the contrary, nerves that are as
tense as bow strings proximal to the constricting tendinous arch,
snapping over the medial epicondyle during elbow flexion and extension,
recover a normal excursion after decompression.
-
Center the incision over the medial
epicondyle, and extend it proximally and distally far enough to expose
all potential compression sites (Fig. 52.40).![]() Figure 52.40. Skin incision for medial epicondylectomy (dark line). The dashed line
Figure 52.40. Skin incision for medial epicondylectomy (dark line). The dashed line
represents a common position of a cutaneous nerve branch (usually from
the medial antebrachial cutaneous), which must be protected during
exposure. (From Heithoff SJ, Millender LH. Medial Epicondylectomy. In:
Gelberman RH, ed. Operative Nerve Repair and Reconstruction. Philadelphia: Lippincott, 1991:1088, with permission.) -
Isolate the ulnar nerve as it enters the cubital tunnel.
-
Decompress the nerve in the cubital
tunnel; lift the retinaculum off the nerve; protect the nerve and
directly visualize it to prevent formation of a painful neuroma. Divide
the retinaculum. -
Trace the nerve into the FCU muscle. Divide the origin of the arch of the FCU.
-
Continue decompression of the nerve between the heads of the FCU; do not damage the multiple muscular branches in this area.
-
Divide the deep flexor pronator aponeurosis until the nerve has been dissected 8 cm distal to the medial epicondyle.
-
Trace the nerve approximately 8 cm proximally.
-
Release the arcade of Struthers.
-
Excise the distal 8 cm of the
intermuscular septum in the arm, taking care not to damage any of the
vessels associated with the nerve. (Multiple vascular leashes exist
near the insertion of the septum into the medial epicondyle.) -
Make an incision directly over the medial
epicondyle. Elevate the flexor pronator mass subperiosteally, both
radially and ulnarly, while preserving the proximal attachments of the
muscle mass (Fig. 52.41A).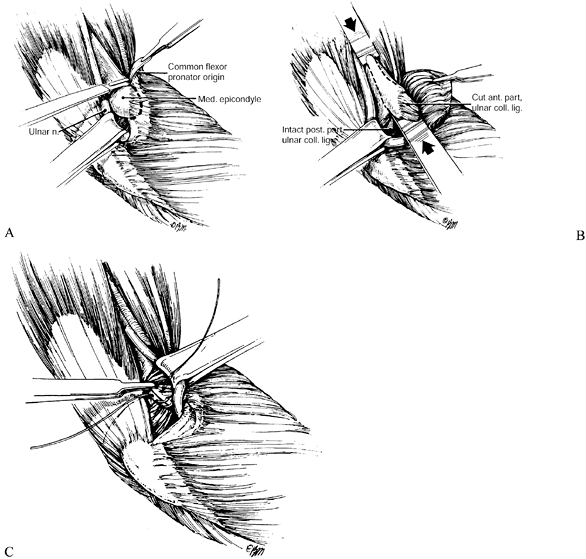 Figure 52.41. Medial epicondylectomy. A: Elevation of the flexor pronator mass. B: Removal of the medial epicondyle with an osteotome. C: Closure of the flexor pronator origin. (From Eversmann WW Jr. Entrapment and Compression Neuropathies. In: Green DP, ed. Operative Hand Surgery, 3rd ed. New York: Churchill Livingstone, 1993:1341, with permission.)
Figure 52.41. Medial epicondylectomy. A: Elevation of the flexor pronator mass. B: Removal of the medial epicondyle with an osteotome. C: Closure of the flexor pronator origin. (From Eversmann WW Jr. Entrapment and Compression Neuropathies. In: Green DP, ed. Operative Hand Surgery, 3rd ed. New York: Churchill Livingstone, 1993:1341, with permission.) -
Perforate the base of the epicondyle
where you will make the osteotomy with a small drill, Kirschner wire
(K-wire), or osteotome. -
Preserve the medial collateral ligament, which arises from the distal, inferior portion of the epicondyle.
-
Remove the epicondyle, cutting it in line with the medial cortex of the distal humerus (Fig. 52.41B). Perforating the planned osteotomy line with drill holes may be helpful.
-
Cover the raw bone surface with bone wax, and close the tendon of the flexor pronator mass (Fig. 52.41C). We recommend decompression of the nerve proximally and distally at all sites of potential compression.
-
Check that the ulnar nerve glides unimpeded during flexion–extension of the elbow.
-
Release the tourniquet and obtain hemostasis.
-
Close only the skin after this.
-
Apply a well-padded dressing and a long arm posterior splint.
Then remove it and begin active range-of-motion exercises. Use a sling
to protect the arm when it is not engaged in therapy. Full range of the
elbow should be achieved by 3–4 weeks. At that time, begin progressive,
gentle strengthening. Theoretically, the nerve will slowly transpose
itself anteriorly and come to rest in a position without tension,
anterior to or on the flexion–extension axis of the elbow. Seradge (112)
demonstrated a decreased incidence of elbow contracture and a decrease
in return-to-work time of 50% in patients who had mobilization of their
elbows within 3 days of surgery following medial epicondylectomy versus
those who were immobilized for 14 days postoperatively.
transposition—subcutaneous, intramuscular, and submuscular—are
essentially similar in their means of initial decompression and
mobilization of the nerve, and this is described here. Both the way the
transposition is maintained and the rehabilitation vary with the
method, however, so they will be described under separate headings.
-
Center the incision between the olecranon
and the medial epicondyle, and extend it along the axes of the humerus
and ulna 8–10 cm proximally and distally. -
Expose the deep fascia, and elevate the
radial flap along this plane over the origin of the flexor–pronator
muscles. Extend the exposure to adequately visualize the ulnar nerve
along its course from the arcade of Struthers to well into the interval
between the heads of the FCU and, laterally, to visualize the median
nerve. -
Isolate the ulnar nerve as it enters the cubital tunnel.
-
Decompress the nerve in the cubital
tunnel; lift the retinaculum off the nerve, protect the nerve and
directly visualize it, then divide the retinaculum. -
Trace the nerve into the FCU muscle. Divide the origin of the arch of the FCU (Fig. 52.42A).
![]() Figure 52.42. Anterior transposition. A: Exposure of the ulnar nerve. B: Decompression carried distally and proximally. C:
Figure 52.42. Anterior transposition. A: Exposure of the ulnar nerve. B: Decompression carried distally and proximally. C:
Excision of the distal 8 cm of the intermuscular septum. (From Spinner
M, Linscheid RL. Nerve Entrapment Syndromes. In: Morrey BF, ed. The Elbow and Its Disorders, 2nd ed. Philadelphia: Saunders, 1993:813; and from Spinner M. Injuries to the Major Branches of the Forearm, 2nd ed. Philadelphia: Saunders, 1978, with permission.) -
Continue decompression of the nerve
between the heads of the FCU (do not damage the multiple muscular
branches in this area), releasing Osborne’s fascia, the fascia of the
two heads of the FCU, and the pronator aponeurosis. -
Divide the deep flexor pronator aponeurosis 8 cm distal to the medial epicondyle.
-
Trace the nerve approximately 8 cm proximally.
-
Release the arcade of Struthers (Fig. 52.42B).
-
Excise the distal 8 cm of the
intermuscular septum of the arm to prevent secondary impingement on the
nerve after anterior transposition. Take care not to damage any of the
vessels associated with the nerve. Multiple vascular leashes exist near
the insertion of the septum into the medial epicondyle (Fig. 52.42C). -
Mobilize the nerve from the cubital tunnel, preserving the small longitudinal vessels accompanying it.
-
You may need to divide small branches
arising from the nerve to the joint; branches to the ulnar head of the
FCU must be preserved. Carefully separate them proximally from the main
trunk by interfascicular dissection, to allow mobilization of the nerve. -
Make sure the nerve can be brought
anterior to the medial epicondyle so that it lies in a relaxed course
anterior to the flexion–extension axis of the elbow, none of the
muscular branches to the FCU are under tension, and there is no kinking
of the nerve proximally or distally.
technique (described next) that employs a fasciodermal sling fashioned
from the fascia of the medial epicondyle and sutured into the dermis of
the anterior skin flap (Fig. 52.43). The nerve
comes to lie in the plane between the dermis and fat of the anterior
flap, and the fascia of the flexor–pronator muscles, and it is
maintained there by the sling. An alternative method for creation of a
sling to maintain the anterior position of the nerve employs the medial
intermuscular septum as described by Pribyl and Robinson (99).
One of us (RMS) avoids the use of fascial slings and prefers to suture
the fat of the lateral skin flap to the medial fascia to create a
broad-based tunnel.
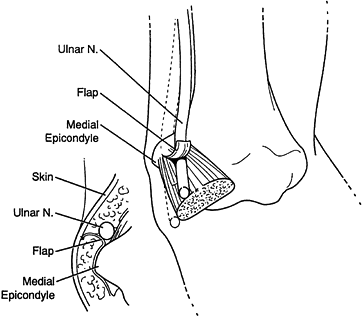 |
|
Figure 52.43.
The fasciodermal sling for maintaining the ulnar nerve in its transposed position. (From Osterman AL, Davis CA. Subcutaneous Transposition of the Ulnar Nerve for Treatment of Cubital Tunnel Syndrome. Hand Clin 1996;12:421, with permission.) |
-
Make a mark on the dermis of the anterior
(radial) skin flap, approximately 1 cm anterior to the medial
epicondyle, to indicate the site of attachment of the dermal sling. -
Raise a strip of fascia from the flexor
pronator muscles at least 1.5 cm in length, detaching it distally so
that it hinges proximally from the medial epicondyle. -
Transpose the nerve anterior to the hinge of the fascial flap.
-
Suture the fascial flap into the dermis
of the anterior skin flap with nonabsorbable suture, at the mark. Take
care to not damage cutaneous branches in the flap arising largely from
the medial antebrachial cutaneous nerve. -
Inspect the nerve in its position
anterior to the fasciodermal sling. The nerve must not bend at the
sling and may only bend at a 45° angle at the FCU hiatus with the elbow
in flexion and extension. It must glide without tethering, and there
must be enough space for a hemostat to be passed alongside it without
difficulty. -
Release the tourniquet and obtain hemostasis.
-
Close the skin.
exercises. Weirich et al. (144)
demonstrated average return to work and activities of daily living by 1
month in patients immediately mobilized after subcutaneous
transposition, versus 2.75 months in those mobilized later than 7 days.
By 2–3 weeks, the patient should regain nearly full range of motion of
the elbow. Progress with strengthening thereafter, with a return to
most activities by 6 weeks postoperatively.
century, and it was evaluated in a series published by Kleinman and
Bishop in 1989 (63), from which the following
description is derived. We do not use this technique as part of routine
treatment for cubital tunnel syndrome.
-
Decompress and mobilize the ulnar nerve (Fig. 52.44A).
Transpose it anteriorly to a position where it will lie without kinking
or tension, and mark or note its position on the fascia of the
flexor–pronator mass (Fig. 52.44B).![]() Figure 52.44. Intramuscular transposition. A: Decompression and mobilization of the ulnar nerve. B: Transposition of the nerve to the flexor pronator fascia. C: Laying the nerve in a trough. D: Closing the fascia over the nerve. (From Kleinman WB. Anterior Intramuscular Transposition. In: Gelberman RH, ed. Operative Nerve Repair and Reconstruction. Philadelphia: Lippincott, 1991:1069, with permission.)
Figure 52.44. Intramuscular transposition. A: Decompression and mobilization of the ulnar nerve. B: Transposition of the nerve to the flexor pronator fascia. C: Laying the nerve in a trough. D: Closing the fascia over the nerve. (From Kleinman WB. Anterior Intramuscular Transposition. In: Gelberman RH, ed. Operative Nerve Repair and Reconstruction. Philadelphia: Lippincott, 1991:1069, with permission.) -
Return the nerve to the ulnar groove, and
make an incision in the fascia of the flexor–pronator musculature where
the nerve lay in the previous step. -
Along this incision, create a 5- to
10-mm-deep trough in the substance of the muscles. Sharply excise
portions of the fibrous septae that cross the trough from within the
bellies of the muscles so that the tissue contacted by the nerve will
be muscle. -
Lay the nerve in the trough. Ensure that
its course is without sharp angles at the proximal and distal extents
of the trough, and that it rests easily in the trough, beneath the
level of the fascia (Fig. 52.44C). -
Close the fascia over the nerve, and test
the excursion of the nerve under the closure; it should glide without
hindrance. Fascial closure is facilitated by placing the forearm in
full pronation and the elbow in 90° of flexion (Fig. 52.44D). -
Release the tourniquet and obtain hemostasis.
-
Close the skin.
-
Place the arm in a bulky splint that holds the forearm in 45° of pronation and the elbow in 90° of flexion.
position and the elbow in flexion for 3 weeks, then institute
progressive range of motion exercises. Permit unrestricted use by 6
weeks.
provided the classical description of the technique of submuscular
transposition of the ulnar nerve. He lengthened the flexor–pronator
muscle mass by a stepcut, or Z-lengthening,
of the common tendinous origin from the medial epicondyle to decrease
the tension on the nerve in its anterior position on the fascia of the
brachialis muscle and anterior capsule of the elbow (89).
-
Identify the lateral border of the flexor–pronator origin, and raise the anterior skin flap adequately to visualize it.
-
Identify the median nerve, which lies
lateral to the flexor–pronator origin. The goal of the procedure is to
place the ulnar nerve alongside the median nerve. -
To gain access to the lateral margin of the muscles, cut the fascial reflection at this margin.
-
Using blunt dissection, elevate the
flexor–pronator muscles from lateral to medial. This dissection begins
distal to the flexor–pronator origin and proceeds between the
flexor–pronator muscle group and the brachialis muscle and anterior
elbow capsule. -
The appropriate medial exit of this
dissection is in the interval between the humeral and ulnar heads of
the FCU. To facilitate completion of the lateral-to-medial dissection
in this plane, identify the interval medially. -
After the entire muscle mass is raised,
detach it from its origin by creating a 90° stepcut in the common
tendon, and turn it distally (Fig. 52.45A).
Leave the posterior limb of the stepcut long and based on the
epicondyle. To avoid damage to the medial collateral ligament complex
of the elbow, you may use an elevator to dissect the muscle off the
anterior elbow capsule. At this stage, ensure that the deep pronator
aponeurosis, which forms a common intermuscular septum between the FCU
and the humeral head of the FDS (Fig. 52.31), is divided adequately to avoid kinking of the nerve distally when it is transposed.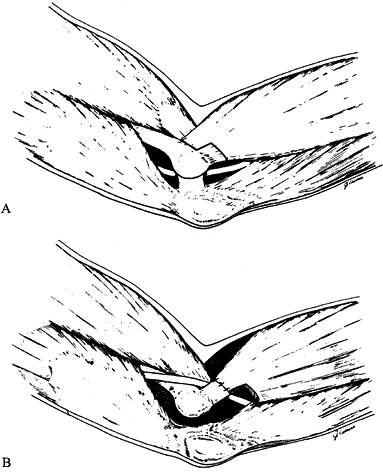 Figure 52.45. Detachment of the flexor pronator mass from the medial epicondyle using a stepcut. A: Making the stepcut. B:
Figure 52.45. Detachment of the flexor pronator mass from the medial epicondyle using a stepcut. A: Making the stepcut. B:
Reattachment of the flexor pronator mass after transposition of the
nerve. (From Rayan GM. Proximal Ulnar Nerve Compression: Cubital Tunnel
Syndrome. Hand Clin 1992;8:325, with permission.) -
Transpose the nerve anteriorly so that it
lies next to the median nerve on the anterior surface of the brachialis
muscle and tendon and the anterior joint capsule, where it will be deep
to the flexor pronator mass. -
Make certain there is no kinking of the
nerve proximally at the arcade of Struthers (or the medial
intermuscular septum) or distally at the deep pronator aponeurosis, and
that the muscular branches of the nerve are not under tension (Fig. 52.46). Protect the posterior branch of the medial antebrachial cutaneous nerve.![]() Figure 52.46. Anterior submuscular transposition. A:
Figure 52.46. Anterior submuscular transposition. A:
Extensile incision, allowing visualization of all potential compression
sites. Note the proximity of the posterior branch of the medial
antebrachial cutaneous nerve. B:
Transposition of the nerve and reattachment of the muscles to the
epicondyle. (From Spinner M, Linscheid RL. Nerve Entrapment Syndromes.
In: Morrey BF, ed. The Elbow and Its Disorders, 2nd ed. Philadelphia: Saunders, 1993:813; and from Spinner M. Injuries to the Major Branches of the Forearm, 2nd ed. Philadelphia: Saunders, 1978, with permission.) -
Release the tourniquet and carefully secure hemostasis.
-
Reattach the stepcut flexor–pronator
origin in a lengthened position over the ulnar nerve with nonabsorbable
suture, and reapproximate the heads of the FCU with absorbable suture (Fig. 52.45B) (25). -
Close the skin.
-
Apply a well-padded dressing from the hand to the upper arm, with a posterior plaster splint.
immobilization for 7–12 days. Then remove the immobilization, place the
arm in a sling, and institute active range-of-motion exercises. The
wrist can be protected from extending with a removable Velcro splint.
Full elbow range is expected by 3 weeks, when the sling is removed.
Restrict the patient from forceful gripping with the hand for an
additional 1–3 weeks, but encourage light activities. Begin
strengthening 4–6 weeks postoperatively, and have the patient return to
nearly full activities at 6–9 weeks, with unrestricted use at 9–12
weeks depending on individual progress and demands.
syndrome are incomplete decompression and inadequate mobilization of
the nerve at the time of transposition. Failure to decompress the nerve
at the site of entrapment often results in failure to relieve symptoms,
and this can be avoided by carefully evaluating all potential sites of
compression. When initial relief of symptoms is followed by recurrence,
common causes are kinking of the nerve proximally at the arcade of
Struthers or on the margin of the intermuscular septum, and distally at
the deep pronator aponeurosis. You can avoid this by making the
incisions adequate for exposure and by examining the nerve at surgery,
before closing the wound, to ensure that it is not entrapped by the
procedure anywhere along its length.
repeat neurodiagnostics, including the inching studies previously
mentioned, to evaluate for the potential site of secondary compression.
When revision surgery is appropriate, the procedure of choice is
submuscular transposition (26) unless this was
the initial procedure. In that case, the choice of revision surgical
technique is often determined at surgery, at which time the status of
the nerve and its bed can be evaluated. The results of repeat
decompression are poor in comparison to those of initial decompression (5,106).
The medial antebrachial cutaneous nerve (particularly the posterior
branch) is at risk for injury during the dissection. This injury
commonly results in pain and an unhappy patient.
the motor, sensory, or motor and sensory portions of the ulnar nerve in
the canal of Guyon. The canal of Guyon is a confined fibro-osseous
triangular space on the ulnar aspect of the volar wrist (Fig. 52.47).
The roof of this space is formed by the volar carpal ligament
proximally (the thickened distal extension of the antebrachial fascia,
which becomes confluent with the tendinous insertion of the FCU onto
the pisiform) and the pisohamate ligament distally (which is the
extension of the FCU from the pisiform onto the hook of the hamate).
The lateral wall is formed by the TCL proximally and the hook of the
hamate distally. The medial wall is formed by the pisiform and its
associated fibrous structures, and the abductor digiti minimi. The
ulnar artery and nerve enter the canal of
Guyon
at the wrist, deep and radial to the FCU tendon, the artery lying
radial to the nerve. In the proximal portion of the canal, the motor
and sensory bundles of the nerve lie side by side as one nerve. Just at
the distal margin of the pisiform, the nerve divides into deep and
superficial branches. The deep branch, which is the motor division,
dives through a fibrous arch formed by the origins of the abductor
digiti minimi and flexor digiti minimi at the base of the hypothenar
muscle group. It continues on to innervate the intrinsic muscles of the
hand. The superficial branch continues on through the canal, beneath
the pisohamate ligament, to course along the palmar surface of the
hypothenar musculature and become, in its terminal branches, the proper
digital nerves of the ulnar and radial small finger and the ulnar ring
finger.
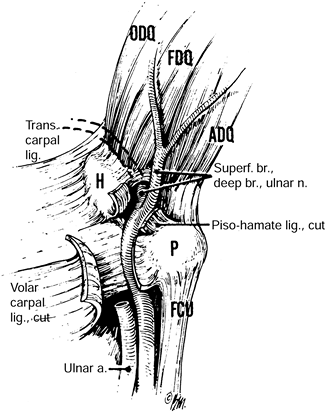 |
|
Figure 52.47.
The anatomy of the ulnar tunnel and the course of the ulnar nerve from the forearm into the wrist as seen in a palmar view of the medial side of the wrist. ODQ, opponens digiti quinti muscle; FDQ, flexor digiti quinti muscle; ADQ, abductor digiti quinti muscle; H, hook of hamate; P, pisiform; FCU, flexor carpi ulnaris tendon. (From Eversmann WW Jr. Entrapment and Compression Neuropathies. In: Green DP, ed. Operative Hand Surgery, 3rd ed. New York: Churchill Livingstone, 1993:1341, with permission.) |
Zone I is the area of the canal from the proximal margin of the volar
carpal ligament to the distal margin of the pisiform—more specifically,
where the nerve bifurcates. It is defined by the presence of both the
motor and the sensory divisions of the ulnar nerve. With division of
the nerve into motor and sensory branches, zones II and III are
defined; the former is associated with the motor and the latter with
the sensory division. These last two divisions run side by side in the
canal up to the level of the hypothenar fibrous arch.
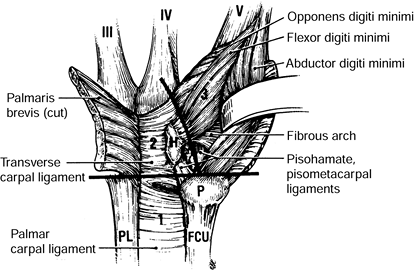 |
|
Figure 52.48.
Zones of the ulnar tunnel. Zone 1 is defined by the presence of both the sensory and motor divisions of the ulnar nerve, prior to the bifurcation at the level of the pisiform. Zone 2 is the zone of the motor branch only. Zone 3 is the region of the sensory branch only. H, hook of hamate; P, pisiform; FCU, flexor carpi ulnaris tendon; PL, palmaris longus tendon. (From Gelberman RH. Ulnar Tunnel Syndrome. In: Gelberman RH, ed. Operative Nerve Repair and Reconstruction. Philadelphia: Lippincott, 1991:1131, with permission.) |
a result of space-occupying lesions. Ganglions arising from the
triquetrohamate joint are the most common cause. Others include
aneurysms (Fig. 52.49), lipomas, fractures of
the bony walls (particularly the hook of the hamate) of the canal of
Guyon, anatomic variations of the canal, and accessory or aberrant
muscles. Repeated blunt trauma to the hypothenar region is the second
most common cause (10,116). Compression in zones I and II is most
often caused by ganglions and fractures of the hook of the hamate. Zone
III is most often affected by a vascular lesion of the ulnar artery (116,128).
 |
|
Figure 52.49. Aneurysm of the ulnar artery within the canal of Guyon (ulnar tunnel), producing an ulnar tunnel syndrome.
|
described three ulnar nerve compression syndromes at and distal to the
wrist. These correspond to the three anatomic areas of the canal. In
type I, the entrapment takes place in the proximal zone, resulting in
mixed motor and sensory deficit. Type II is purely motor, secondary to
compression in the canal of Guyon or at the hook of the hamate in
association with the origins of the abductor digiti minimi and the
flexor digiti minimi and within the substrate of the opponens digiti
quinti. Type III is caused by pressure on the superficial branch of the
ulnar nerve, producing a sensory deficit in the ring and small fingers
without associated muscle weakness or atrophy (Table 52.13).
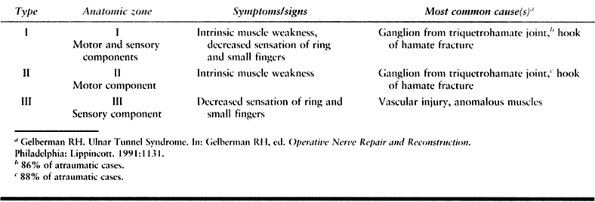 |
|
Table 52.13. Classification of Ulnar Tunnel Syndrome
|
from cubital tunnel syndrome. One difference is the sparing of
sensation of the dorsal ulnar half of the hand in ulnar tunnel
syndrome. This area is innervated by the dorsal branch of the ulnar
nerve, which arises variably from the ulnar nerve, but always proximal
to the wrist crease, and does not enter the canal. Another difference
is sparing of the long flexors of the ring and small fingers, leading
to a more pronounced ulnar claw deformity in patients with ulnar tunnel
versus cubital tunnel syndrome. Additionally, a history of repeated
trauma to the hypothenar region may direct special attention to
evaluation of this area in patients who present with
ulnar-nerve-related symptoms (Table 52.14).
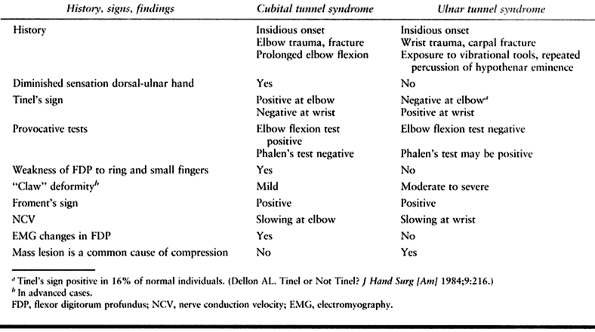 |
|
Table 52.14. Cubital versus Ulnar Tunnel
|
localizing the site of compression. Palpation of the deep structures
within the hypothenar region may suggest a mass, not infrequently a
ganglion within the canal. Allen’s test (an evaluation of the
contributions made by the radial and ulnar arteries to the blood supply
of the hand) is performed because thrombosis or aneurysm of the ulnar
artery may be the cause of the neuropathy. Tinel’s test over the ulnar
nerve at the level of the wrist and compression of the nerve over zone
I may be helpful in distinguishing ulnar tunnel from cubital tunnel
syndrome, although this has not been verified by studies. Phalen’s test
may also be positive in ulnar tunnel syndrome.
and fractures associated with compression of the ulnar nerve in the
canal of Guyon, obtain radiographs. Plain radiographs, including PA,
lateral, and carpal tunnel (ulnar tunnel) views, can be used to screen
for fractures,
dislocations,
and arthritis. If a hook-of-the-hamate fracture is suspected but not
proven in plain radiographs, a CT scan is the diagnostic study of
choice. Mass lesions include ganglia and aneurysms and are best
evaluated by MRI. In some cases of vascular lesions, arteriograms may
be of additional help. Electrodiagnostic studies show slowing of the
NCV across the wrist of the sensory and/or motor portions of the nerve.
In theory, isolated slowing in the sensory portion is not diagnostic of
isolated sensory division compression because the sensory fibers of the
nerve are more susceptible to compression than are the motor fibers. If
EMG evaluation shows abnormalities in the FCU and FDP muscles in
addition to the intrinsics, compression of the ulnar nerve is present
proximally (Table 52.15).
 |
|
Table 52.15. Ulnar Tunnel Syndrome Summary
|
in which cessation of trauma may lead to complete resolution of
symptoms, and for the mild type II neuropathy, the ulnar tunnel
syndrome is best treated surgically. In the absence of identifiable
lesions causing compression of the nerve in the ulnar tunnel,
nonoperative treatment consists of splinting, NSAIDs, and avoidance of
activities that aggravate or precipitate symptoms. Failure to
adequately improve symptoms is an indication for surgery.
tunnel syndrome are treated operatively because there is often a mass
lesion or a vascular injury or anomaly causing compression of the
nerve. In cases of acute onset of ulnar tunnel syndrome in association
with wrist trauma and swelling, Vance and Gelberman (140) recommend operative release and exploration if no improvement is noted within 48 hours. Alternatively, Howard (52)
suggests observation for 6–8 weeks after the injury before exploration.
It is reasonable to treat acute, traumatic ulnar tunnel syndrome
expectantly in cases of incomplete or mild neurologic deficit, and to
reserve urgent operative decompression for those cases with dense
sensory deficits and/or paralysis of intrinsic muscles. Results of
operative management are generally good, and recurrence is rare. Return
of muscle function is adequate to avoid the need for tendon transfer.
The rate and extent of recovery are likely related to the preoperative
duration and severity of symptoms.
essential to treat the offending lesion at surgery. Plan for and inform
the patient of excision of a fractured hook of the hamate or arthritic
pisiform. Vascular reconstruction may
be
necessary in ulnar artery aneurysms and thrombosis. Ganglia must be
identified and excised. If no particular lesion is identifiable,
suspicion of a particular zone of compression will help direct
particular attention to the segment of the nerve corresponding to that
zone.
according to the particular pathology suspected as the cause of
compression. The technique of exploration and decompression of the
nerve through its course in the ulnar tunnel is described here.
-
Center a zigzag incision over the canal
of Guyon, beginning just proximal to the proximal wrist crease at the
radial border of the FCU tendon (Fig. 52.50).
Or, in the case of concomitant CTS, use the incision for carpal tunnel
release described earlier in this chapter and carry the dissection
ulnarly to expose the canal of Guyon.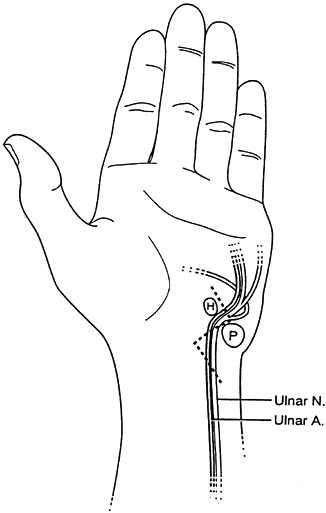 Figure 52.50.
Figure 52.50.
Incision for exposure of the ulnar tunnel. H, hook of hamate; P,
pisiform. (From Osterman AL, Kitay GS. Compression Neuropathies: Ulnar.
In: Peimer CA, ed. Surgery of the Hand and Upper Extremity. New York: McGraw-Hill, 1996:1339, with permission.) -
Begin dissection proximally. Incise the fascia along the lateral border of the FCU.
-
Identify the ulnar artery and nerve after retracting the FCU medially; the artery lies lateral to the nerve.
-
Trace the ulnar nerve and artery
distally, and incise (in order) the overlying palmaris brevis muscle,
the hypothenar fat, any fibrous bands, and the palmar carpal ligament. -
Trace the sensory branch onto the hypothenar muscles, dividing any crossing and potentially constrictive structures.
-
Trace the motor branch into the fibrous
arch of the hypothenars and several millimeters distally, again
dividing any potentially constricting fibrous structures. -
Inspect the floor, walls, and contents of
the canal, and identify any masses, aberrant muscles, fractures, or
offending structures. -
Identify and inspect the ulnar artery for any abnormalities.
-
One of us (JT) performs release of the carpal tunnel routinely at this time.
-
Close the skin.
-
Apply a padded hand dressing similar to that for carpal tunnel release.
you treated other pathology simultaneously, institute specific
rehabilitation and advise precautions as indicated.
Incomplete release is potentially a problem if the nerve is not traced
distally enough into zone III, between the pisohamate and
pisometacarpal ligaments. An additional pitfall is failure to recognize
the existence of a vascular component within this syndrome.
lateral intermuscular septum in the arm (most frequently in conjunction
with displaced fractures of the humerus) or compressed proximal to the
elbow in association with a fibrous arch from the lateral head of the
triceps (a rare form of compression) (75).
Evaluation of these two conditions reveals involvement of the muscles
innervated by the posterior interosseous nerve as well as those by the
radial nerve prior to its branching into motor and sensory
divisions—typically the brachioradialis and extensor carpi radialis
longus (ECRL) muscles. Additionally, sensory disturbances in the radial
nerve distribution are common with these disorders. EMG evaluation is
often diagnostic, and observation is the treatment of choice. If
recovery is not spontaneous within 3 months, exploration and
decompression are warranted.
Additionally, the superficial, sensory branch of the radial nerve (SBR)
can be compressed in the distal forearm, causing pain and sensory
disturbances in the radial nerve sensory distribution.
the middle third of the humerus in a diagonal course between the
lateral and medial heads of the triceps. At the distal third of the
humerus, the nerve pierces the lateral intermuscular septum and then
travels anteriorly and distally between the brachialis and
brachioradialis muscles. Just proximal to the elbow, the nerve
bifurcates into superficial and deep branches. The superficial branch,
the sensory division, continues distally, traveling between the
brachioradialis and supinator muscles. The posterior interosseous
(deep) branch, essentially a purely motor nerve, passes between the two
heads of the supinator muscle under an inverted fibrous arch, the
arcade of Frohse, which is formed by the thickened edge of origin of
the superficial head and is seen readily (Fig. 52.51) (55). It continues beneath the supinator muscle, exiting under its
distal edge. The PIN innervates the muscles of the posterior forearm (Fig. 52.52).
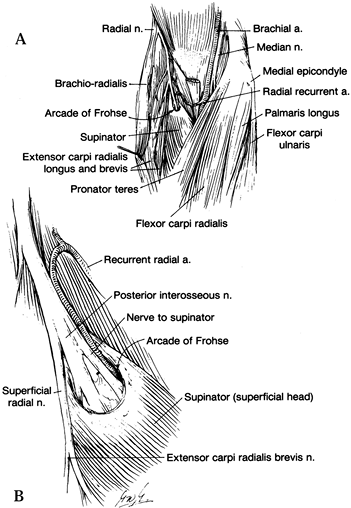 |
|
Figure 52.51. A: Composite drawing of dissections of the forearm at the level of the elbow. B:
Enlarged view of the posterior interosseous nerve and its relationship to the supinator muscle. The motor supply to the extensor carpi radialis brevis arises most frequently from the superficial radial nerve. (From Spinner M. Injuries to the Major Branches of Peripheral Nerves of the Forearm. Philadelphia: Saunders, 1972, with permission.) |
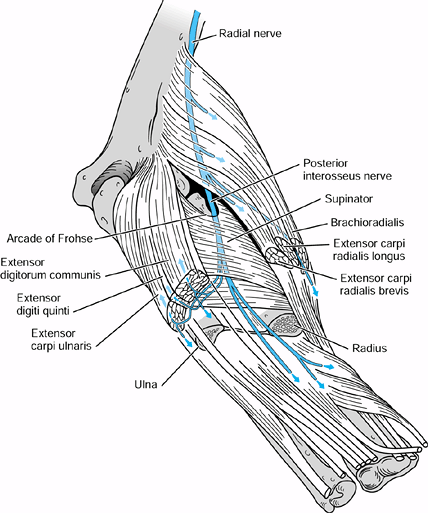 |
|
Figure 52.52.
Innervations of the posterior interosseous nerve. Not labeled in the diagram are the distal innervations, from radial to ulnar: abductor pollicis longus, extensor pollicis brevis, extensor pollicis longus, and terminal sensory branches to the dorsal carpus. (From Spinner M, Linscheid RL. Nerve Entrapment Syndromes. In: Morrey BF, ed. The Elbow and Its Disorders, 2nd ed. Philadelphia: Saunders, 1993:813; and from Spinner M. Injuries to the Major Branches of the Forearm, 2nd ed. Philadelphia: Saunders, 1978, with permission.) |
Just proximal to the radiocapitellar joint, after branching from the
radial nerve, the PIN travels through fibrous tissue that is associated
with the anterior capsule of the radiocapitellar joint. This fibrous
tissue may form bands that can cause constriction of the nerve (37).
The nerve continues distally and is crossed by the leash of Henry,
small radial recurrent vessels that have been reported to be a source
of compression (70). The fibrous leading edge
of the extensor carpi radialis brevis (ECRB) muscle travels diagonally
across the proximal edge of the supinator muscle and can cause
compression of the PIN just prior to its entrance under the arcade of
Frohse (70,107,118).
The edge of the ECRB and the fibrous arcade of Frohse, the thickened
proximal edge of the supinator muscle, are the most common sites of
compression in PIN syndrome (Table 52.16). The
nerve wraps around the proximal radius between the two heads of the
supinator and may also be compressed as it exits from beneath the
superficial head on its distal margin of this muscle.
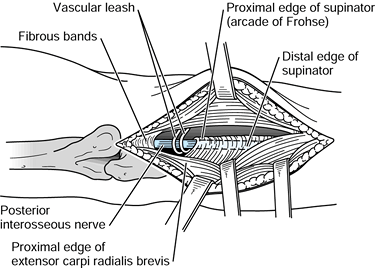 |
|
Figure 52.53.
The five potential compression sites of the posterior interosseous nerve: fibrous bands on the anterior radiocapitellar joint capsule, the vascular leash of Henry (radial recurrent vessels), the proximal edge of the extensor carpi radialis brevis, the arcade of Frohse (proximal edge of the supinator), and the distal edge of the supinator. (From Hynes DE, Peimer CA. Compression Neuropathies: Radial. In: Peimer CA, ed. Surgery of the Hand and Upper Extremity. New York: McGraw-Hill, 1996:1291, with permission.) |
 |
|
Table 52.16. The Five Potential Compression Sites of the PIN from Proximal to Distal
|
PIN compression may also be iatrogenic, as after internal fixation of
fractures of the proximal radius, in which the nerve is extremely
vulnerable because it may lie directly on the periosteum opposite the
tuberosity of the radius (Fig. 52.54) (120).
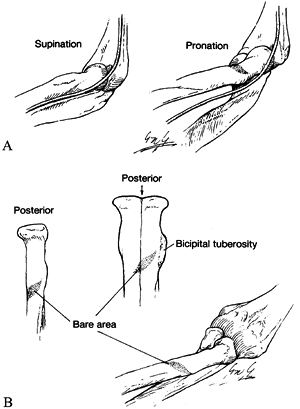 |
|
Figure 52.54. A:
In supination with a bare area of the proximal radius, the posterior interosseous nerve comes to lie against the periosteum of the radius. B: Details of the bare area. (From Spinner M. Injuries to the Major Branches of Peripheral Nerves of the Forearm. Philadelphia: Saunders, 1972, with permission.) |
insidious. There may be a history of trauma to the radial head or neck.
Symptoms include weakness of the PIN-innervated muscles, and pain but
not sensory dysfunction. The bra-chioradialis and the ECRL are not
involved because they are innervated by the radial nerve. The ECRB may
be spared because its innervation may originate more proximally, or it
may appear to arise from the superficial bifurcation
of the radial nerve (Fig. 52.51).
The complete syndrome involves loss of extension of all digits and of
the extensor carpi ulnaris. The patient can dorsiflex the wrist but
with radial deviation. Middle and distal digital joints may still be
extended through their intact intrinsics. Partial syndromes may involve
just the extensor digitorum communis (EDC), with extension of the thumb
and index finger preserved (Fig. 52.55). Early
in the compression syndrome, a less striking clinical picture may
exist, with paresis or paralysis of isolated digits. Patients with
rheumatoid
arthritis
may present the same attitude of the hand whether the cause is a PIN
compression or rupture or dislocation of the EDC tendons. A careful
examination assists the clinician in differentiating between these two
entities (76,78).
 |
|
Figure 52.55. Incomplete dorsal interosseous nerve syndrome.
|
elbow, especially in cases of trauma or arthritis. Soft-tissue masses
may stretch or compress the PIN near the proximal radius. Functional
loss of finger extension may be present, even though the patient is
completely unaware of the presence of a mass. MRI examinations are
extremely helpful in these patients, for preoperative evaluation of the
size, shape, and location of the mass. Electrodiagnostic studies are
imperative. The EMG portion of the examination is usually diagnostic (Table 52.17).
 |
|
Table 52.17. Posterior Interosseous Nerve (PIN) Syndrome
|
in cases of trauma. In cases of fracture or dislocation, follow
management algorithms for treatment of such injuries complicated by
nerve dysfunction. In the rare case of acute onset of PIN without
injury, rule out the presence of a mass. In the absence of fracture,
dislocation, or tumor, the patient can be evaluated with EMG studies
and observed for 6–8 weeks. During this time, we recommend NSAIDs,
maintaining joint mobility to prevent contracture, and avoidance of
activities requiring repetitive or forceful elbow or wrist flexion and
extension and forearm pronosupination. Splinting of the wrist in slight
extension alone will often improve function of the hand markedly.
Additional dynamic splinting for MP joint extension aids in opening the
hand for grasp, but the splint itself may be cumbersome and interfere
with activities. Failure to improve clinically or electromyographically
in 6–8 weeks is an indication for operative treatment.
Patients generally recover motor function, although improvement may
take 18 months to complete. If the paralysis has been longstanding
(more than 12–18 months), so that end organ innervation of the muscle
has atrophied, there will be residual loss or inadequate recovery.
These patients can be treated with appropriate tendon transfers (see Chapter 55 on radial nerve palsy) (54).
dislocations. You must delineate the sizes and locations of masses to
optimize the choice of approach. This requires a preoperative MRI. In
cases of suspected malignancy, perform a metastatic workup and choose a
treatment appropriate for the neoplasm. In patients with rheumatoid
arthritis and radiocapitellar disease, radial head excision may be
indicated.
proximal forearm. Use the one that allows complete visualization of the
potential compression sites of the nerve and that allows treatment of
any associated conditions. Three popular approaches are the
anterolateral extensile, posterior, and brachioradialis
muscle–splitting approaches. Perform the surgery under tourniquet
control and regional or general anesthetic.
-
Begin the incision 5 cm above the flexor
crease on the lateral aspect of the arm, over the interval of the
brachioradialis and biceps. Extend it distally, across the flexor
crease, along the ulnar border of the brachioradialis muscle (Fig. 52.56).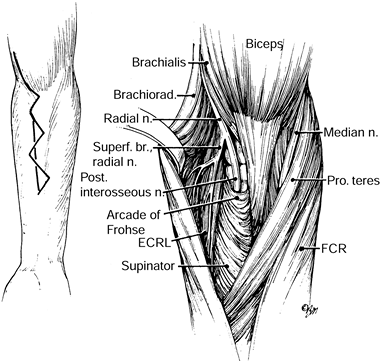 Figure 52.56.
Figure 52.56.
The anterolateral approach to the radial nerve. (From Eversmann WW Jr.
Entrapment and Compression Neuropathies. In: Green DP, ed. Operative Hand Surgery, 3rd ed. New York: Churchill Livingstone, 1993:1341, with permission.) -
Develop the skin flaps to the level of
the muscle fascia. Protect the lateral brachial and lateral
antebrachial cutaneous (LABC) nerves as well as the cephalic vein in
the subcutaneous tissues. -
Deepen the dissection proximally and
identify the radial nerve in the interval between the brachialis and
brachioradialis just proximal to the flexion crease of the elbow. -
Trace the nerve distally onto the
anterior capsule of the radiocapitellar joint, the first site of
potential compression, where fibrous bands may compress the nerve.
Release all substantial structures crossing the nerve. -
Continue tracing the nerve distally. The
fan-shaped leash of vessels from the radial recurrent artery cross the
nerve as they travel to the brachioradialis. With bipolar cautery,
coagulate the vessels and divide them. -
While visualizing the nerve, pronate the
forearm and flex the wrist. This will tighten the leading edge of the
ECRB and may demonstrate its compression of the PIN. Remove a portion
of the fibrous edge of the muscle in the region of the nerve. -
Continue following the nerve as it enters the arcade of Frohse. Divide the fibrous arch while protecting the underlying PIN.
-
The superficial head of the supinator
often has a tough fascial envelope. Because of this and for the benefit
of exploration of the nerve as it travels through the supinator, it may
be preferable to divide the superficial head of the supinator as it
crosses the PIN. -
Trace the nerve as it branches and exits the supinator. Divide the distal margin of the superficial head of the supinator.
-
Make an incision along the line
connecting the lateral epicondyle and Lister’s tubercle with the
forearm in pronation. Begin it 2 cm distal to the lateral epicondyle,
and extend it 8 cm. -
Locate the interval between the ECRB and
EDC muscles in the distal portion of the wound. Develop this interval
and extend it proximally. -
Identify the transverse-oblique fibers of the supinator muscle (Fig. 52.57).
![]() Figure 52.57.
Figure 52.57.
The posterior muscle-splitting approach to the radial tunnel and the
arcade of Frohse. ECU, extensor carpi ulnaris; ECRB, extensor carpi
radialis brevis; ECRL, extensor carpi radialis longus; EDC, extensor
digitorum communis. (From Eversmann WW Jr. Entrapment and Compression
Neuropathies. In: Green DP, ed. Operative Hand Surgery. New York: Churchill Livingstone, 1982:1341, with permission.) -
Increase exposure by developing the
interval between the ECRB and the supinator. Avoid dissection between
the EDC and the supinator, which would place the motor innervation of
the EDC at risk (120). -
Explore the nerve as previously
described, beginning proximally. It may be difficult to isolate the
fibrous bands of the first site of compression, which is a disadvantage
of this approach. (One can extend this approach proximally by
developing the interval on the medial and lateral aspects of the
brachioradialis muscle.)
-
Make an incision centered over the mobile wad at the level of the radiocapitellar joint (Fig. 52.58).
 Figure 52.58. Incision for the muscle-splitting approach to the radial tunnel.
Figure 52.58. Incision for the muscle-splitting approach to the radial tunnel. -
Develop the skin flaps to the level of
the muscle fascia. Protect the lateral brachial and LABC nerves in the
subcutaneous tissues. -
Make an incision in the brachioradialis fascia in line with the muscle fibers.
-
Palpate the radial head.
-
Bluntly dissect through the substance of
the muscle, separating its fibers and heading toward the radial head.
Continually locate the radial head by palpation. -
Identify the transverse-oblique fibers of
the supinator. The superficial branch of the radial nerve will be on
the underside of the brachioradialis (Fig. 52.59).![]() Figure 52.59.
Figure 52.59.
The muscle-splitting approach to the radial tunnel. (From Peimer CA,
Wheeler DR. Radial Tunnel Syndrome/Posterior Interosseous Nerve
Compression. In: Szabo RM, ed. Nerve Compression Syndromes: Diagnosis and Treatment. Thorofare, NJ: Slack, 1989:177, with permission.) -
Identify the PIN as it enters the arcade of Frohse.
-
Dissect the nerve, evaluating the five
potential sites of compression and dividing all potential offending
structures. (Pronate the forearm and flex the wrist to evaluate ECRB
compression of the nerve.) Carry decompression out to the distal margin
of the supinator. -
If more proximal exposure is desired,
extend the skin incision along the interval between the brachioradialis
and the biceps on the arm. Proceed with deeper exposure as described in
the anterolateral approach. -
If more distal exposure is desired,
extend the incision so that you can reach the interval between the ECRB
and EDC muscles. Proceed with deeper dissection as described in the posterior approach. -
Loosely approximate the fascia of the brachioradialis before closing the skin.
-
Start the incision 4–5 cm proximal to the
elbow flexion crease, at the interval between the brachioradialis
laterally and the brachialis medially. -
Distally, continue the incision to the interval between the ECRB and the ECRL laterally and to the EDC medially (Fig. 52.60).
 Figure 52.60. Approach for the radial nerve and its bifurcation at the elbow and proximal forearm.
Figure 52.60. Approach for the radial nerve and its bifurcation at the elbow and proximal forearm. -
Begin deep dissection at the proximal end of the incision.
-
Identify the radial nerve and tag it with a moist, wide Penrose drain.
-
At the distal end of the incision,
develop the plane between the ECRB and the EDC, exposing the oblique
fibers of the supinator muscle (Fig. 52.57). -
Accomplish wider exposure by partially detaching the ECRB from the lateral epicondyle.
-
Dissection to increase exposure is
between the ECRB and the supinator, as in the posterior approach. (Do
not dissect between the EDC and the supinator.) -
Trace the nerve from proximal to distal, using the proximal and distal intervals for exposure.
-
Divide all potential compressing structures as previously noted.
and obtain hemostasis. Do not close any deep structures except as noted
for the brachioradialis-splitting technique. Suture the skin in layers.
Apply a padded dressing and a long-arm splint with the forearm in
supination and with the elbow flexed 90°.
finger range-of-motion exercises. Because these approaches are carried
out between anatomic planes, you may discontinue immobilization as
early as 3–5 days and no later than 7–10 days. Depending on individual
patient recovery, slings or wrist splints may be used for up to 3 weeks
for comfort. Formal therapy for strengthening and range of motion is
not routinely implemented but should be used when the patient is slow
to progress. Return-to-work time is influenced by the severity of the
syndrome and the particular job demands.
syndromes, differentiation of the PIN syndrome from extensor tendon
ruptures or dislocations in the patient with rheumatoid arthritis is
important. Make this distinction by performing a tenodesis test. When
the wrist is passively flexed, the fingers will not extend at the MP
joints if the tendons are ruptured. If the tendons are intact, as in
PIN syndrome, extension of the MP joints will occur as the wrist falls
into flexion.
and the extensor digitorum, because this will jeopardize the
innervation of the EDC muscle. Identification and resection of a
portion of the fibrous leading edge of the ECRB where it crosses the
PIN, as well as separate division of the arcade of Frohse, are critical
because these are the common sites of compression of the nerve.
“Recurrence,” or rather failure of operative treatment, is caused by
incomplete decompression of the nerve at all the potential sites of
entrapment.
radial nerve and its posterior interosseous (deep) branch, beginning
proximally where the radial nerve lies deep between the brachioradialis
and the brachialis, and extending distally to the distal border of the
supinator muscle (107). The five sites of compression are the same as those for PIN syndrome (97,107), easily remembered by the mnemonic FREAS (Table 52.16).
Unlike in PIN syndrome, the compression of the PIN in RTS is not caused
by masses, fractures, and disturbances of the radiocapitellar joint. It
is associated with repetitive elbow flexion and extension and forearm
rotation. It is likely a dynamic form of compression of the PIN (57).
motor deficit and usually without sensory changes. Patients present
with a dull, aching or burning pain over the lateral aspect of the
elbow in the region of the proximal extensor–supinator muscle mass.
Symptoms may radiate distally along the course of the extensor muscles
to the radial aspect of the hand or proximally to the shoulder. In some
cases, there may be paresthesias in the radial nerve distribution.
Symptoms are worsened by activities, especially those requiring
forceful and repetitive elbow motion or wrist flexion and extension and
forearm pronosupination, and are relieved by rest. The association
between lateral epicondylitis, or “tennis elbow,” and RTS has been
noted (107,141).
Patients may present with tennis elbow symptoms and evidence of lateral
epicondylitis, which may respond to local steroid injections followed
by localization of discomfort several centimeters distal to the lateral
epicondyle over the radial tunnel. The resultant RTS can then exist
solely or concurrently with the tennis elbow; 5% of patients with
lateral epicondylitis also have RTS (128). The diagnosis of RTS is differentiated from tennis elbow by examination.
over the radial head and neck along the course of the radial nerve.
Comparison of the opposite, unaffected side is recommended because many
normal individuals find this area exquisitely tender to deep palpation.
Distal radiation of symptoms can occur with manual compression of
the
nerve in this region. Limitation of elbow extension and weakness of
grip further suggest RTS. Sensory deficits in the distribution of the
superficial radial branch may be seen in RTS and suggest compression of
the nerve in the proximal aspect of the tunnel at a point where both
divisions of the main trunk are simultaneously vulnerable, such as
beneath the fibrous bands proximal to the arcade of Frohse. Because of
its dynamic or exertional nature, provocative testing is invaluable in
detecting RTS and may give clues to the site of compression of the
nerve within the radial tunnel. Described provocative tests for RTS
include the elbow flexion test, the middle finger extension test (107), passive pronation of the forearm (33), and the supination test (70) (Table 52.18). In the elbow flexion test (Fig. 52.61),
ask the patient to flex his elbow against resistance. Reproduction of
pain with this maneuver indicates compression of the nerve by fibrous
bands on the capsule of the radiocapitellar joint. In the middle finger
extension test (Fig. 52.62), ask the patient to
extend his middle finger against resistance applied by you over the
proximal phalanx. Hold his forearm in full pronation and his elbow
extended. Reproduction of pain in the radial tunnel region with this
maneuver indicates entrapment of the PIN at the ECRB tendon.
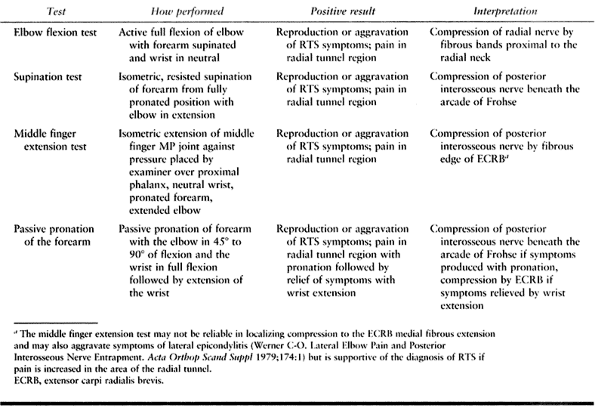 |
|
Table 52.18. Provocative Tests for Radial Tunnel Syndrome (RTS)
|
 |
|
Figure 52.61. The elbow flexion test for radial tunnel syndrome.
|
 |
|
Figure 52.62. The middle finger extension test for radial tunnel syndrome.
|
diagnosis of RTS. Conduction velocities are often normal, and EMG
changes are present in patients with clinically evident weakness or
atrophy and therefore carry the diagnosis of PIN syndrome. There may be
a place for dynamic electrodiagnostic studies to detect subtle changes
in nerve function (64,100).
We believe that a localized injection of anesthetic, with or without
steroid, into the radial tunnel, which produces a PIN palsy and
relieves symptoms, is diagnostic for RTS. Localized steroid injection
may also assist in differentiating RTS from tennis elbow (Table 52.19).
 |
|
Table 52.19. Radial Tunnel Syndrome Summary
|
wrist splinting, administration of NSAIDs, and avoidance of activities
that involve forearm pronosupination. Prescribe formal hand therapy.
Administer steroid iontophoresis over the radial tunnel three times per
week for 2–3 weeks. After significant reduction in inflammation, begin
soft-tissue mobilization, gentle progressive stretching, and nerve
gliding exercises. Institute strengthening later, tailoring it so that
it does not exacerbate symptoms.
treatment, progressive symptoms, and the impracticality of permanent
activity modification are all indications for surgical intervention.
complete resolution of pain may take several months to a year. Lister et al. (70)
noted approximately 92% good to excellent results in a review of the
combined cases of three series, including their own. Jebson and Engber (57) reported 71% good results and 29% fair/poor results in their 8-year follow-up of 33 extremities in 31 patients. Ritts et al. (105)
reviewed the results of 34 cases performed over the span of 10 years.
They noted only 51% good results with surgery, and workers’
compensation cases often had an unsatisfactory result. There is no
difference in the operative results of patients with normal
preoperative electrodiagnostic studies and those with abnormal ones.
Peimer and Wheeler (97) noted that the best results were in patients whose nerves appeared normal at surgery. Ritts et al. (105) noted that the best prognostic factor for operative treatment was a good response to a preoperative injection.
same as that described for PIN syndrome. Because of its simplicity and
sufficient exposure, the brachioradialis–splitting technique (107)
is preferred. If the site of compression has been well localized by
clinical methods to the arcade of Frohse, a limited skin incision may
be employed.
-
Make an incision approximately 6 cm long, starting at the elbow crease and projected longitudinally over the radial head (Fig. 52.58).
-
Expose the brachioradialis and split its
fibers by blunt dissection. Take care to not injure branches of the
lateral brachial and antebrachial nerves. -
After traversing the brachioradialis,
retract the superficial branch of the radial nerve and the ECRL
laterally to expose the deep branch of the radial nerve and the oblique
fibers of the supinator (Fig. 52.59, Fig. 52.63).![]() Figure 52.63. Superficial and deep branches of the radial nerve. A: The deep branch is seen as it passes under the arcade of Frohse (arrow). B: Compression of the deep branch (arrow) after division of the superficial head of the supinator.
Figure 52.63. Superficial and deep branches of the radial nerve. A: The deep branch is seen as it passes under the arcade of Frohse (arrow). B: Compression of the deep branch (arrow) after division of the superficial head of the supinator. -
Divide the structures at the five potential sites of compression to fully expose the PIN.
-
Loosely approximate the deep fascia.
-
Close the skin.
range-of-motion exercises. Remove the postoperative dressing at 5 days.
Place the arm in a sling and the wrist in a cock-up splint. Encourage
active range-of-motion exercises of the elbow and wrist so that full
range is achieved by 3 weeks postoperative. Remove the wrist splint and
sling at 3 weeks and institute therapy for elbow and wrist range of
motion if the patient is progressing slowly. If the patient is
progressing well, encourage progressive use of the upper extremity in
daily activities, but caution against forceful activities until 6 weeks
after surgery. If job demands are fairly strenuous, a strengthening
program may be prescribed under a therapist’s supervision. Return to
work is allowed at 6–8 weeks but may not be realized until
up
to 3–4 months for patients who are manual laborers. Complete recovery
can take up to a year. Job modification in conjunction with surgery may
be indicated, especially in workers’ compensation cases.
examination and must not be overdiagnosed. Overdiagnosis leading to
incorrect treatment or overtreatment is the main cause of failure.
Lateral epicondylitis is far more common. Injections of local
anesthetic over the lateral epicondyle and more distally over the
radial tunnel are helpful in differentiating between these two entities
and in establishing the diagnosis. When the two conditions coexist and
are unresponsive to nonoperative management, treatment of the lateral
epicondylitis at the time of radial tunnel release is requisite for
maximal postoperative relief of symptoms. Postoperatively, transitory
but distressing partial paresis of the finger extensors may occur, but
it resolves spontaneously in 6–12 weeks.
He suggested that the condition be named cheiralgia paresthetica
because of its similarity to the isolated involvement of the lateral
cutaneous nerve of the thigh, which is called meralgia paresthetica.
in the interval between the brachioradialis and brachialis muscles. The
superficial sensory branch continues toward the wrist under cover of
the brachioradialis and medial to the ECRL muscle. It courses
superficially to the supinator in the proximal forearm, and then to the
PT insertion in the midforearm. In the distal third of the forearm, the
nerve pierces the antebrachial fascia between the tendons of the
brachioradialis and ECRL muscles. The nerve passes superficially to the
extensor retinaculum and divides into terminal branches to provide
cutaneous innervation to the dorsoradial aspect of the hand (Fig. 52.8).
lies deep to the fascia, without compression from the tendons of the
brachioradialis and ECRL muscles. As the forearm pronates, the ECRL
tendon crosses beneath the brachioradialis tendon in a scissors-like
fashion, pinching or compressing the nerve (Fig. 52.64A). Palmar–ulnar deviation of the wrist places traction on the nerve (Fig. 52.64B).
Repetitive traumatization of the nerve causes swelling, which inhibits
its normal gliding through the fascia, leading to a traction injury (27).
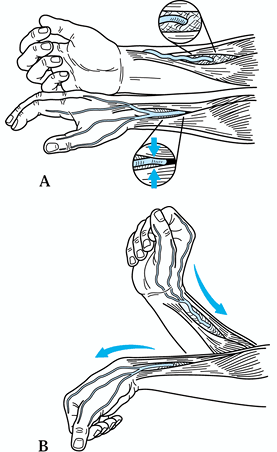 |
|
Figure 52.64. A: Pronation of the forearm causes a pinching of the superficial branch of the radial nerve. B:
Palmar–ulnar flexion of the wrist puts the superficial radial nerve in maximal traction. (From Dellon AL, MacKinnon SE. Radial Sensory Nerve Entrapment in the Forearm. J Hand Surg [Am] 1989;11:199, with permission.) |
pronosupination is given. Affected patients complain of pain, numbness,
tingling, and dysesthesias over the dorsoradial aspect of the hand.
Symptoms are brought on by wrist movement and are intensified when the
patient makes a tight grip with the thumb and index finger. Nighttime
awakening caused by symptoms is not common. Sensory examination reveals
alterations in moving two-point and vibratory sensation. There is no
motor dysfunction of radially innervated muscles, although grip and
pinch strength may be decreased secondary to pain. Percussion along the
course of the nerve, particularly as it emerges between the
brachioradialis and ECRL tendons, produces paresthesias. Dellon and
MacKinnon (27) described a provocative test for
eliciting symptoms in which the patient is instructed to pronate the
forearm with the elbow in extension. If within 30–60 seconds the
symptoms of paresthesias or dysesthesias are evoked or exacerbated over
the dorsal radial aspect of the hand, entrapment is confirmed (Fig. 52.65).
 |
|
Figure 52.65.
The provocative test for entrapment of the superficial branch of the radial nerve, consisting of forced pronation of the forearm. (From Dellon AL, MacKinnon SE. Radial Sensory Nerve Entrapment in the Forearm. J Hand Surg [Am] 1989;11:199, with permission.) |
radial nerve entrapment is de Quervain’s stenosing tendovaginitis of
the first dorsal wrist compartment. Finklestein’s test (i.e., pain on
quick ulnar deviation of the hand with the patient’s thumb grasped in
the palm) is positive in both entities. The presence or absence of
swelling over the first dorsal compartment, a nerve compression test
over the course of the superficial radial nerve, and careful sensory
testing are used to differentiate these two entities. Electrodiagnostic
testing is rarely useful, although it often shows abnormalities in
conduction velocity or amplitude (27). Injury
to the LABC nerve as a source of the patient’s symptoms must also be
excluded because there is overlap in the innervation of the LABC and
the SBR nerves in the mid and distal forearm in 75% of patients (27).
Exclude it by performing serial blocks with local anesthetic. First
block the LABC nerve at the lateral arm, then block the SBR at the site
of suspected entrapment. Improvement in symptoms subsequent to the
second injection but not the first implicates the SBR as the cause (Table 52.20).
 |
|
Table 52.20. Cheiralgia Paresthetica Summary
|
pronosupination and radioulnar deviation of the wrist, splinting of the
wrist with or without inclusion of the thumb and forearm, and NSAIDs.
Physiotherapy, including tissue mobilization and nerve gliding
exercises, steroid iontophoresis, and progressive gentle stretching,
may be helpful. Administer steroid injections subfascially, and use
them judiciously
because
of the potential for depigmentation of the skin and subcutaneous fat
atrophy. Patients with long-term symptoms or onset of symptoms
associated with a fracture or crush injury tend not to improve with
nonoperative treatment.
pain relief was good or excellent in 86% of patients undergoing
surgery. Of these patients, 43% returned to their regular jobs, 22%
returned to modified work, and 35% remained disabled because of
associated injuries. Objectively, marked improvement was noted in grip
and pinch strength.
-
Make an 8 cm curvilinear incision just
volar to the mid-radial aspect of the radius, centered in relation to
the point where the nerve pierces the antebrachial fascia (Fig. 52.66A).
(The location of the positive Tinel’s sign may also be used.) To
prevent scarring about the nerve, avoid placing the incision directly
over the nerve.![]() Figure 52.66. Decompression of the superficial branch of the radial nerve. The incision (A) is represented by the dashed line. The fascia joining the brachioradialis and extensor carpi radialis longus (B) is divided distally (C) and proximally (D).
Figure 52.66. Decompression of the superficial branch of the radial nerve. The incision (A) is represented by the dashed line. The fascia joining the brachioradialis and extensor carpi radialis longus (B) is divided distally (C) and proximally (D).
The nerve is lying loosely in its bed, and the brachioradialis and ECRL
are gliding independently. (From Dellon AL, MacKinnon SE. Radial
Sensory Nerve Entrapment in the Forearm. J Hand Surg [Am] 1989;11:199, with permission.) -
Identify and protect cutaneous branches of the LABC nerve.
-
Identify the SBR and the fascia between the BR and ECRL.
-
Release the fascia joining the BR and ECRL distally out to the insertion of the BR (Fig. 52.66B).
-
Release the fascia at least 6 cm proximal to the nerve (Fig. 52.66C).
-
Ensure that the nerve lies loosely in the
subcutaneous tissue and that the BR and ECRL glide independently
without constriction of the SBR (Fig. 52.66D). -
Close the wound, and apply a bulky compression dressing with a volar splint.
discourage splinting. Begin a home program of scar therapy and tissue
mobilization. Encourage the patient to use the hand progressively in
regular activities.
accurate diagnosis and good surgical technique will prevent
complications. You must differentiate between SBR entrapment and de
Quervain’s tendovaginitis by physical examination. If concomitant de
Quervain’s exists, treat it appropriately. Exclusion of entrapment of
the LABC nerve by differential anesthetic blocks is also imperative.
Avoid excessive handling of the nerve at surgery to prevent neuroma
formation.
musculocutaneous nerve. Arising from the musculocutaneous nerve in the
interval between the biceps and brachialis muscles, it continues
distally under cover of the biceps muscle and then the lateral border
of the biceps tendon to the elbow flexion crease. At this level, the
nerve lies on top of the brachialis, lateral to the biceps and medial
to the brachioradialis. It pierces the brachial fascia lateral to the
biceps tendon. It divides into an anterior branch and a posterior
branch, the former traveling with the cephalic vein to innervate the
anterior surface of the radial forearm to the thenar eminence, and the
latter continuing to innervate
the radial and posterior aspect of the distal forearm to the wrist (Fig. 52.67). The anterior branch of the LABC nerve communicates with the superficial radial nerve above the wrist (8).
Compression of the LABC nerve is rare but can occur where it emerges
from beneath the lateral border of the biceps tendon just medial to the
brachioradialis. The nerve is compressed between the brachialis fascia
and the tendon of the biceps when the elbow is extended. Compression of
the nerve can be further accentuated with pronation of the forearm (8,91).
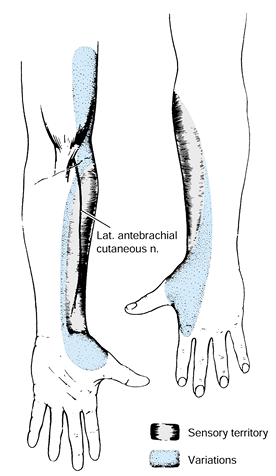 |
|
Figure 52.67.
Palmar and dorsal sensory innervation of the lateral antebrachial cutaneous (musculocutaneous) nerve. (From Nunley JA, Howson P. Lateral Antebrachial Nerve Compression. In: Szabo RM, ed. Nerve Compression Syndromes: Diagnosis and Treatment. Thorofare, NJ: Slack, 1989:201, with permission.) |
forceful exercise of the elbow in a position of extension. Patients
complain of pain over the lateral aspect of the elbow on active motion
of the elbow, with accompanying burning or numbness in the radial
forearm. Examination reveals decreased sensibility distally along the
radial aspect of the forearm. Elbow extension is limited with the
forearm fully pronated. Point tenderness is found lateral to the biceps
tendon at the elbow crease. Compression may be confirmed with sensory
NCV studies measured between the elbow flexion crease and the axilla. A
nerve block with local anesthetic that produces numbness along the
nerve’s cutaneous distribution and eliminates symptoms aids in the
diagnosis. The nerve lies between the cephalic and median cubital veins
1.5 cm lateral to the biceps tendon, and the injection is best placed
just distal to the cubital crease (Table 52.21).
 |
|
Table 52.21. Lateral Antebrachial Cutaneous (LABC) Nerve Compression
|
patients with a diagnosis of LABC nerve entrapment. Average follow-up
was 13.4 years, with a minimum of 2 years. Eleven of these patients
underwent surgical decompression. Of these, none had recurrence of
hypesthesia, and all had complete relief of pain and full range of
motion. One patient subsequently underwent release of the lateral
epicondyle. Of the four patients who were treated nonoperatively, one
had persistent hypesthesia but full range of motion and complete pain
relief.
to rule out bony pathology. Examine the patient on more than one
occasion to confirm consistent findings. Consider all extraarticular
and intraarticular elbow conditions when evaluating the patient.
-
Begin the incision proximal to the elbow
flexion crease along the lateral border of the biceps muscle. Curve it
laterally at the elbow flexion crease to head toward the radial tunnel.
Do not cross the cubital flexion crease at right angles. -
Identify the LABC nerve 1.5 cm lateral to the tendon at the level of the medial condyle (Fig. 52.68A).
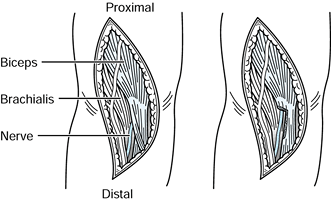 Figure 52.68. A: The lateral antebrachial nerve emerging from beneath the lateral border of the biceps tendon. B:
Figure 52.68. A: The lateral antebrachial nerve emerging from beneath the lateral border of the biceps tendon. B:
A wedge-shaped section taken out of the overlying biceps tendon to
decompress the nerve. (From Nunley JA, Howson P. Lateral Antebrachial
Nerve Compression. In: Szabo RM, ed. Nerve Compression Syndromes: Diagnosis and Treatment. Thorofare, NJ: Slack, 1989:201, with permission.) -
Trace the nerve proximally several centimeters.
-
Demonstrate the area of entrapment by
pronating the arm in extension. Look for flattening of the nerve and
loss of vascular markings. -
Excise a triangular wedge of biceps tendon (1×3 cm) where the nerve is compressed by the edge of the biceps and the brachialis (Fig. 52.68B).
-
Ensure that the decompression is complete by pronating and supinating the elbow while it is in extension.
-
Apply a bulky dressing, and splint the elbow at 90° in neutral rotation.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
JM, McCarroll HR, Jr., Tortosa RD, et al. Endoscopic Release of the
Carpal Tunnel: A Randomized Prospective Multicenter Study. J Hand Surg [Am] 1992;17:987.
RR, Evanoff BA, Chough LY, et al. The Use of Routine Wrist Radiography
in the Evaluation of Patients with Carpal Tunnel Syndrome. J Hand Surg [Am] 1997;22:115.
J, Beck J, Gillet J. Provocative Motor Nerve Conduction Testing in
Presumptive Carpal Tunnel Syndrome Unconfirmed by Traditional
Electrodiagnostic Testing. J Hand Surg [Am] 1997;22:1045.
DL, Watson HK, Caulfield KA, et al. Persistent or Recurrent Carpal
Tunnel Syndrome Following Prior Endoscopic Carpal Tunnel Release. J Hand Surg [Am] 1998;23:1010.
RH, Pfeffer GB, Galbraith RT, et al. Results of Treatment of Severe
Carpal Tunnel Syndrome without Internal Neurolysis of the Median Nerve.
J Bone Joint Surg Am 1987;69:896.
SJ, Millender LH, Nalebuff EA, et al. Medial Epicondylectomy for the
Treatment of Ulnar Nerve Compression at the Elbow. J Hand Surg [Am] 1990;15:22.
JW. General Principles and Use of Electrodiagnostic Studies in Carpal
and Cubital Tunnel Syndromes: With Special Attention to Pitfalls and
Interpretation. Hand Clin 1996;12:205.
DL, Staebler MP, Weiss A-PC, Akelman E. The Results of Revision Carpal
Tunnel Release Following Previous Open versus Endoscopic Surgery. J Hand Surg [Am] 1998;23:865.
CD, Sybert DO, Albarracin NS. An Analysis of the Flexor Synovium in
Idiopathic Carpal Tunnel Syndrome: Report of 625 Cases. J Hand Surg [Am] 1992;17:1028.
WE, Follender AB. Interfascicular Neurolysis in the Severe Carpal
Tunnel Syndrome. A Prospective, Randomized, Double-blind, Controlled
Study. Clin Orthop 1988;227:251.
SE, McCabe S, Murray JF, et al. Internal Neurolysis Fails to Improve
the Results of Primary Carpal Tunnel Decompression. J Hand Surg [Am] 1991;16:211.
BS. A Simple Clinical Test to Differentiate Rupture of Flexor Pollicis
Longus and Incomplete Anterior Interosseous Paralysis. J Hand Surg [Br] 1992;17:510.
K, Shinatro T. Histology of the Transverse Carpal Ligament and Flexor
Tenosynovium in Idiopathic Carpal Tunnel Syndrome. J Hand Surg [Am] 1998;23:1015.
PA, Keniston RC, Lockwood RS, Meadows KD. Tobacco, Caffeine, Alcohol
and CTS in American Industry. A Cross Sectional Study of 1464 Workers. J Occup Environ Med 1996;38:290.
P, Keniston R, Myers L, et al. Obesity as a Risk Factor for Impaired
Sensory Conduction of the Median Nerve in Industry: A Cross-sectional
and Longitudinal Study Involving 429 Workers. J Occup Med 1992;34:117.
PA, Keniston RC, Myers LD, Meadows KO. Obesity as a Risk Factor for
Slowing of Sensory Conduction of the Median Nerve in Industry. A Cross
Sectional and Longitudinal Study Involving 429 Workers. J Occup Med 1992;34:379.
P, Meadows K, Keniston R. Rehabilitation of Carpal Tunnel Surgery
Patients Using a Short Surgical Incision and an Early Program of
Physical Therapy. J Hand Surg [Am] 1993;18:1044.
R, Kleinert JM. Ulnar Nerve Decompression by Transposing the Nerve and
Z-Lengthening the Flexor-Pronator Mass: Clinical Outcome. J Hand Surg [Am] 1997;22;127.
CR, Robinson B. Use of the Medial Intermuscular Septum as a Fascial
Sling During Anterior Transposition of the Ulnar Nerve. J Hand Surg [Am] 1998;23:500.
JG, Barnes K, Gelberman RH, Chalidapong P. Endoscopic Carpal Tunnel
Release: An Anatomic Study of the Two-Incision Method in Human
Cadavers. J Hand Surg [Am] 1992;17:996.
MA, Gelberman RH, Gellman H, Rhoades CE. Carpal Tunnel Syndrome:
Associated Abnormalities and Ulnar Nerve Function and the Effect of
Carpal Tunnel Release on These Abnormalities. J Hand Surg [Am] 1985;10:710.
Heest AE, House J, Krivit W, Walker K. Surgical Treatment of Carpal
Tunnel Syndrome and Trigger Digits in Children with Mucopolysaccharide
Storage Disorders. J Hand Surg [Am] 1998;23:236.
SD, Gelberman RH, Best SA, et al. Rehabilitation after Subcutaneous
Transposition of the Ulnar Nerve: Immediate Versus Delayed
Mobilization. J Shoulder Elbow Surg 1998;7:244.
DH, Krout R. Surgery of Ulnar Neuropathy at the Elbow: 16 Cases Treated
by Decompression without Transposition. Technical Note. J Neurosurg 1973;38:780.
L, Dellon AL. Brachial Neuritis Presenting as Anterior Interosseous
Nerve Compression—Implications for Diagnosis and Treatment: A Case
Report. J Hand Surg [Am] 1997;22:536.