Proximal Humerus Fractures
debilitating injuries and are an increasing problem in the elderly.
There is universal agreement that most stable fractures, which often
occur in frail, elderly patients, are best treated nonoperatively. The
major controversy surrounds the minority of more complex, displaced and
multipart fractures. More ink than blood may have been spilt in the
debate over these injuries, and their discussion in the orthopaedic
literature is disproportionate to their prevalence. As a consequence,
patients with similar injuries may receive widely different opinions
about the severity of their fracture, its likely outcome, and its best
treatment, dependent on the unit in which they are treated and the
surgeon who treats them.
difficulty in assessing these injuries. There are substantial
difficulties in classifying these injuries reliably and reproducibly
and in evaluating their outcome. There is also considerable variation
in the treatment expectations and likely outcome for different
patients, dependent on their age and functional capabilities before
their injury. There is a wide range of treatment options for these
injuries, each with its advantages and disadvantages. It may be
difficult to reconcile the risk of complications from one particular
form of treatment against its likely benefits. Finally, over the past
10 years, there has been considerable expansion
in
the range of reconstructive implants available to treat these injuries.
Their uncontrolled introduction, without clear evidence of superiority
over existing techniques, further confounds any attempt at rational
appraisal of relative merits of the different treatment options.
of the existing literature have highlighted the paucity of Level I or
II studies of these injuries. The need for prospective, randomized
multicenter clinical trials comparing the available treatment options
is clear, yet few such studies are ongoing at present. This lack of
high-level scientific evidence confounds any attempt at
consensus-based, protocol-driven management. The aims of this chapter
are therefore to describe the assessment tools and treatment options
that are currently available for these injuries and to highlight the
major areas of controversy in their management.
osteoporotic fractures, with an annual incidence of between 63 and 105
fractures per 100,000 population per year.57,129,165,187,225,260 They account for 5% of all injuries to the appendicular skeleton.55,187 The prevalence of these fractures is increasing in the elderly,139,225
although whether this is a direct result of the shifting population
demographic, or an age/sex-specific rise is unclear. The current
exponential rise in incidence is set to continue, with one projection
suggesting a trebling of the number of fractures in the elderly over
the next three decades.225
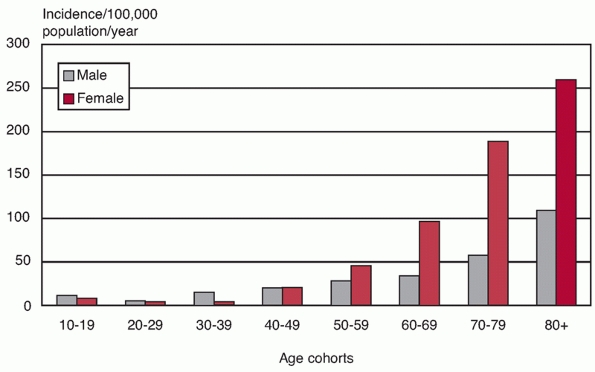
elderly distribution curve with a low incidence under the age of 40
years and an exponential increase thereafter.55 Although most patients are typically in the younger aging population (aged between 65 and 75 years),55,57,274 the skewed population demographics dictate that the age-/sex-adjusted incidence of fractures in the elderly is much higher57,129,165,187,225,260 (Fig. 35-1). There are marked gender differences, with approximately 70% to 80% of fractures occurring in women,55,57,165,187 in studies from North America, Scandinavia, the United Kingdom, France, southern Europe, Japan, and Australia.11,57,109,129,187,226,260,268
These fractures are less common in the Japanese population compared
with that of northern Europe or North America and are also less common
in black Americans compared with white Americans.10,109,260
The majority of fractures are undisplaced or stable two-, three-, and
four-part fractures, and a detailed evaluation of their prevalence is
provided in the classification section of this chapter.
 |
|
FIGURE 35-1
The age/gender-specific incidence of proximal humeral fractures in an urban population. (Data from CourtBrown CM, Garg A, McQueen MM. The epidemiology of proximal humeral fractures. Acta Orthop Scand 2001;72: 365-371.) |
produced by high-energy injuries, mainly from road traffic accidents,
sports injuries, falls from height, or gunshot wounds. However, these
are much less common than fractures in the elderly, which are usually
low-energy osteoporotic injuries.13,57,165,181,187 More than three quarters follow low-energy domestic falls,57,165,187,260
and the risk of fracture is increased in sedentary individuals with low
bone mineral density, a family history of osteoporotic fracture,
frequent falls, and evidence of impaired balance.174
Middle-aged patients who sustain low-energy fractures frequently have a
predisposing medical comorbidity or are physiologically older through
the effects of alcohol, drug, or tobacco overuse.212,213
Any other condition that produces osteoporosis at an earlier age will
also increase the risk of fracture; in females, an early menopause is
probably the most common cause of this.
is thought to fracture on the hard-packed bone of the glenoid, which
acts as an anvil.73 The interaction
of this external force with the forces generated by the intrinsic
shoulder musculature, and the quality of the proximal humeral bone
stock, determines the initial fracture configuration and any ensuing
displacement. Elderly patients, with advanced osteoporosis or with
medical comorbidities, are more likely to have displaced fractures.222
A proximal humeral fracture may occur from direct impact to the
shoulder or indirectly by transmission of forces from a fall onto the
outstretched arm. Depleted protective neuromuscular responses, because
of a delayed reaction time, cognitive impairment, neuromuscular
disorders, impaired balance, or acute intoxication, increase the risk
of a fall directly onto the shoulder.174,226,264 The nondominant arm is also affected in up to
three quarters of cases,196,324
suggesting an association with reduced strength and neuromuscular
coordination. Diminished protective responses are an indirect measure
of poor physiologic status, and this may explain why patients who
sustain proximal humerus fractures from direct impact on the shoulder
tend to be frailer than those who sustain wrist fractures,142,222,274 where the arm is outstretched to break the fall.
pathologic from metastatic tumor deposits or, rarely, caused by a
primary bone tumor or infection. In contrast, persistence of shoulder
pain after a significant injury with normal radiographs may be caused
by an occult fracture (typically of the greater tuberosity) or a
rotator cuff injury.237,317 This may only be detectable using ultrasound or magnetic resonance imaging (MRI).237,317
appendicular skeleton and is prone to instability. Fracture-dislocation
is therefore more common in this area than in other juxta-metaphyseal
fractures. The diaphysis expands into the surgical neck, which is just
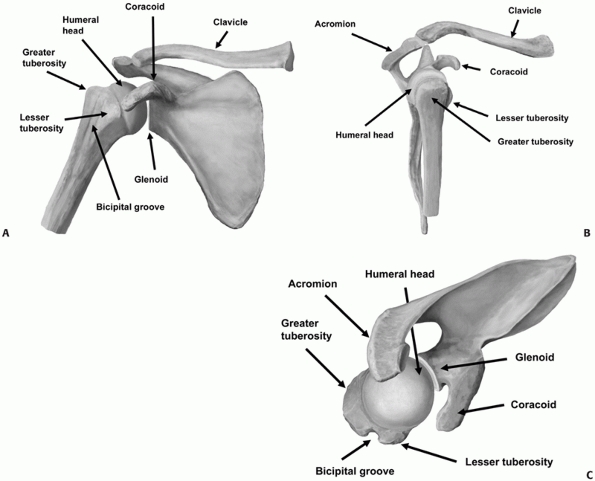
below the greater and lesser tuberosities at the metaphyseal flare (Fig. 35-2).
The anatomic neck is the region immediately above the tuberosities and
below the humeral articular surface. The humeral articular segment
occupies approximately one third of a sphere, with a diameter of
curvature averaging 46 mm (with a range of 37 to 57 mm).29,124,131
The inclination of the humeral head relative to the shaft averages 130
degrees (with a range of 123 to 136 degrees), and the geometric center
of the humeral head is offset an average of 2.6 mm posteriorly (range
of -0.8 to 6.1 mm) and 7 mm (range of 3 to 11 mm) medially from the
axis of the humeral shaft.29,124,131,243
The humeral head is normally retroverted by an average of 20 degrees,
with respect to the distal humeral interepicondylar axis. However, the
degree of retroversion may vary quite dramatically from between 10
degrees of anteversion to 60 degrees of retroversion.29,124,167
 |
|
FIGURE 35-2 The bony anatomy of the proximal humerus viewed from (A) anteriorly, (B) laterally, and (C) superiorly.
|
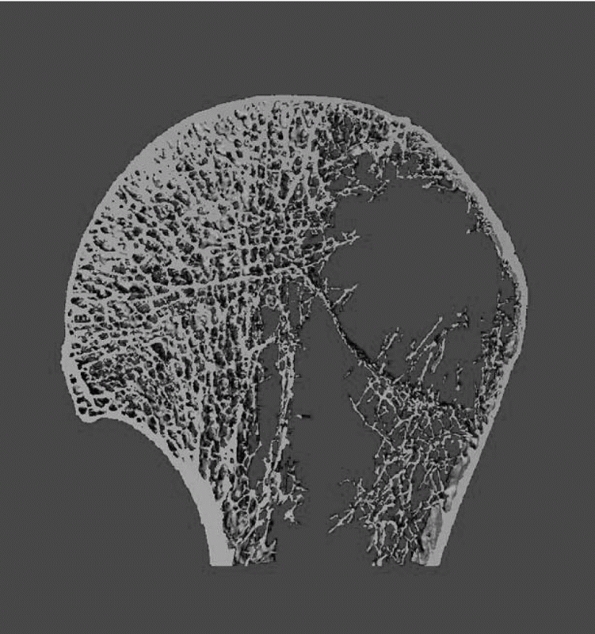
that the distribution of bone within the proximal humerus is not
uniform. The subchondral bone beneath the articular surface is
predictably dense cancellous bone, with bone mineral density decreasing
progressively toward the geometric center of the humeral head and into
the metaphyseal area of the surgical neck265 (Fig. 35-3).
The overall bone quality of the proximal humerus can be reliably
predicted by the cortical thickness of the proximal diaphysis,293 as well as the age of the patient.313
Because most proximal humeral fractures occur from compressive and
shear forces, these areas of relative bone paucity are prone to
impaction, creating cancellous defects when the fracture is reduced.
These may be filled by the use of bone grafts or bone graft substitutes.106,169,253 The highest bone density is found in
the subchondral bone immediately beneath the articular surface. There
is also a progressive decrease in bone mineral density from superior to
inferior and from posterior to anterior in the four quadrants of the
humeral head.120,293,294,295
The head is therefore analogous to a hen’s egg, with a strong,
compressionresistant exterior and a less mechanically robust interior.
Knowledge of these areas of bone concentration is important to
appreciate when operative plate or nail reconstructive techniques are
used, because poor screw positioning in the head may compromise the
rigidity of the surgical reconstruction, leading to early failure.
 |
|
FIGURE 35-3
Microcomputed tomographic study of cancellous trabeculae in humeral head demonstrates marked porosity in greater tuberosity region and most dense bone just underneath humeral head. (Reprinted with permission from Meyer DC, Fucentese SF, Koller B, Gerber C. Association of osteopenia of the humeral head with full-thickness rotator cuff tears. J Shoulder Elbow Surg 2004;13:333-337.) |
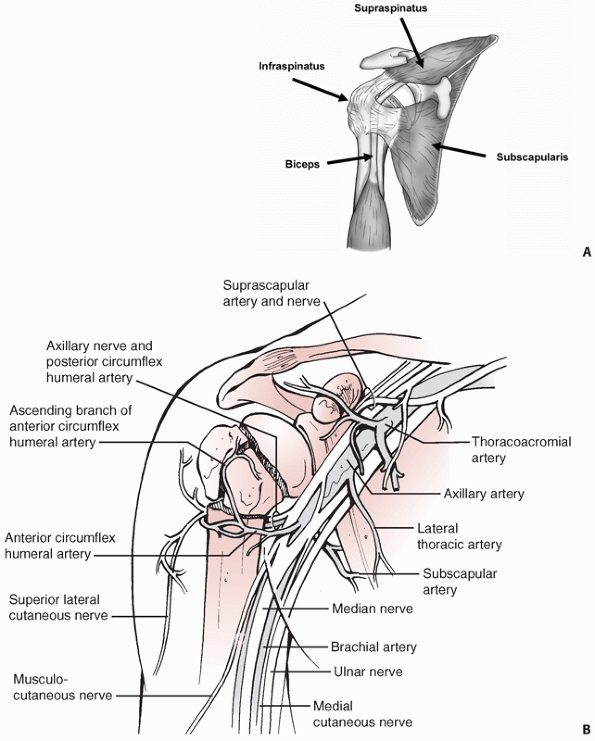
periscapular musculature, and rotator cuff. The latter consists of the
musculotendinous units of subscapularis, supraspinatus, infraspinatus,
and teres minor. The tendinous long head of the biceps in its groove
separates the lesser from the greater tuberosity. It is therefore a
valuable surgical landmark for identifying these fracture fragments,
and it serves as a “tramline” to the terminus at the superior pole of
the empty glenoid in fracture-dislocations (Fig. 35-4).
The subscapularis tendon inserts into the lesser tuberosity, which is
separated from the greater tuberosity by the bicipital groove. The
displacement of the tuberosities when they are fractured is governed by
their soft tissue attachments. The greater tuberosity is typically
pulled medially, superiorly, and posteriorly by supraspinatus,
infraspinatus, and teres minor tendons, whilst the lesser tuberosity is
displaced anteriorly and medially by subscapularis. The pull of the
rotator cuff muscles on their tuberosity attachments also explains why
the humeral head is usually displaced posteriorly in a three-part
greater tuberosity fracture, as a result of the unopposed pull of the
subscapularis, whereas the head may be anteverted in the more uncommon
three-part lesser tuberosity fracture. If the periosteal attachments to
the head are ruptured, the humeral shaft may be translated medially
into the axilla, by the pull of the pectoralis major and latissimus
dorsi tendons. Fracture of a tuberosity fragment defunctions the
rotator cuff muscles that attach to it, and the tendon will regain
function only once the fracture has healed. A tuberosity fragment that
becomes displaced and heals with malunion may produce longer-term
dysfunction of the attached cuff tendons or may give rise to
subacromial or coracoid impingement.105,206
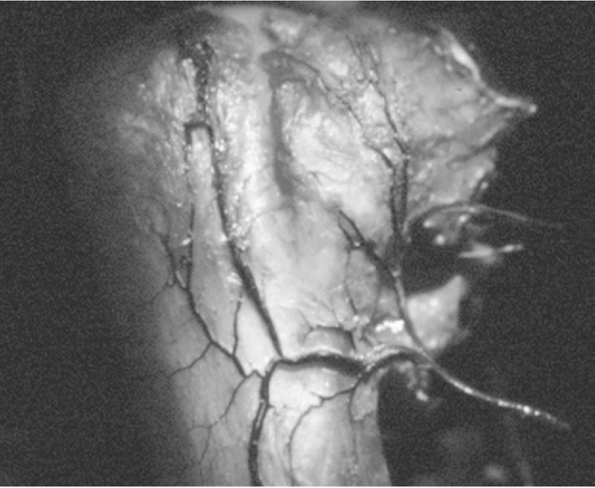
arises from the axillary artery via its anterior and posterior
circumflex humeral branches, which anastamose medially in the
quadrilateral space, laterally in the area of the greater tuberosity,
and in the humeral head through the rich network of interosseous
anastamose (Fig. 35-5). The anterior circumflex
artery contributes collateral branches, which feed the lesser
tuberosity and the humeral head by entering along the line of anterior
and inferior capsular reflection at the anatomic neck. The main branch
of the anterior circumflex is an anterolateral ascending artery, which
ascends the bicipital groove and enters the head just below the
articular surface, to form the intraosseous arcuate artery. In the
uninjured state, this provides the majority of the vessels that perfuse
the humeral head, with the exception of the greater tuberosity and
posteromedial aspect of the head, which are perfused by branches of the
posterior circumflex artery.35,104,172
This provides a leash of vessels, which enter the head along the line
of the capsular insertion in the anatomic neck posteriorly and
inferiorly.
fracture, and alternate sources of vascularization to the humeral head
may become important after injury. The observations that the humeral
head may be perfused and that osteonecrosis does not inevitably occur
after more complex three- and four-part fractures in which these
vessels are damaged lend support to this argument.14,103,123
It is likely that after fracture, additional sources of head perfusion
may come from either of the tuberosities (if they are not fractured),
via their soft tissue capsular and rotator cuff attachments, and from
residual attachments of the capsule to the articular margin. In
particular, the branches of the posterior circumflex humeral artery35,71,194
that enter the head posteroinferiorly along the line of capsular
reflection may maintain arterial perfusion to the head. These vessels
are often intact in three- and four-part valgus fractures of the
anatomic neck, provided the capsule remains intact and the medial
periosteal hinge, which these vessels traverse and which connects the
head to the shaft, has not been damaged.71,123
Fractures of the anatomic neck where the head fragment has an
appreciable intact spike of bone extending into the medial metaphysis
are also at lower risk of osteonecrosis.14,35,71,123,194
This may be because the fracture line is below the line of capsular
reflection and the branches of the posterior circumflex artery are
preserved. Extra-articular surgical neck fractures also have a low risk
of osteonecrosis, because sources from both the anterior and posterior
circumflex vessels are likely to be intact. In contrast, in certain
three- and four-part fractures and fracture-dislocations, where the
capsular attachments are completely disrupted, the risk of
osteonecrosis is much higher.
of the fracture to preserve this important source of blood supply. It
is also important to avoid extensive dissection and soft tissue
stripping, to reduce the risk of damaging other intact periosteal
feeding vessels.
 |
|
FIGURE 35-4 Soft-tissue anatomy of shoulder showing the rotator cuff musculature (A) and the neurovascular structures (B).
|
The trunks, divisions, cord, and branches of the plexus may be damaged
by displaced fracture fragments or through traction injury. During
operative treatment, most of these structures are usually protected
from injury by the conjoined tendons of the short head of biceps and
coracobrachialis, which mark the medial extent of surgical exposure
through the deltopectoral approach. However, it is important to avoid
prolonged traction on these tendons intraoperatively, as this may
injure the musculocutaneous nerve. This pierces the conjoined tendon
approximately 5 to 8 cm below the tip of the coracoid process.80
The axillary nerve (C5-C6) is the main structure at risk during
operative treatment of proximal humeral fractures. The nerve arises
from the posterior cord of the plexus and travels posterolaterally over
the lower subscapularis to enter the quadrilateral space, where it is
an immediate inferior relation of the glenohumeral joint capsule. It
gives off a posterior branch that supplies the posterior deltoid and
teres minor and provides sensation to the “badge area” of the upper
arm. The anterior branch winds around the surgical neck deep to the
deltoid muscle and has a somewhat variable course.153 It innervates the anterior and middle thirds of the deltoid but has no cutaneous branches.
swelling, and bruising of the upper arm, which is usually severe in the
first 2 weeks postinjury. Since the fracture lies deep to the shoulder
musculature, the revealed bruising and swelling are often most
pronounced as it tracks anteroinferiorly into the lower arm. Although
the degree of swelling is a poor marker of the severity
of
the underlying bony injury, very severe swelling may denote an
underlying vascular injury. Open fractures of the proximal humerus are
relatively uncommon, although off-ended fractures with severe anterior
displacement of the proximal shaft may occasionally produce pressure
necrosis of an area of skin in the upper arm, leading to skin breakdown
and infection. Careful reinspection of the anterior soft tissues is
important in this group of patients, as the postinjury swelling settles.
 |
|
FIGURE 35-5
The major blood supply to the humeral head is derived from the anterior humeral circumflex artery. Its ascending branch is shown coursing lateral to the bicipital groove. (From Gerber C, Schneeberer AG, Vinh TS. The arterial vascularization of the humeral head. An anatomical study. J Bone Joint Surg Am 1990;72A:1486-1494, with permission.) |
given the proximity of the adjacent axillary vessels and the tethering
of the surgical neck to the trifurcation of the two circumflex vessels
and the subscapular artery. It is important to carefully assess the
circulation distal to the fracture in every case.232,321
In the presence of a vascular injury, only minimal and subtle signs of
peripheral ischemia may be present, because of the rich collateral
circulation. These are frequently missed initially, but a large or
expanding hematoma, pulsatile external bleeding, unexplained
hypotension, and an associated nerve trunk or plexus injury should
increase the level of suspicion. Axillary arterial laceration may be
produced by the displaced sharp edge of the medial shaft in a displaced
two-part surgical neck fracture. Injury to the vessels is also
relatively common in three- or four-part anterior
fracture-dislocations, where direct injury to the vessels may be caused
by either the displaced humeral head or the displaced shaft. Finally,
intimal tears may be produced by injuries in which there is significant
traction on the arm.
extremity or ongoing signs of vascular compromise should be referred
for specialist vascular surgical advice. Doppler arterial pulse volume
recordings may be useful, and a single-injection trauma angiography is
usually accurate in the operating room setting. If more time is
available, then a formal retrograde femoral arteriogram should be
obtained. In addition, more sophisticated studies including digital
subtraction angiography are now available to assess these injuries
Most neurologic injuries are either direct injuries to the brachial
plexus from fracture fragments (by the same mechanisms as for vascular
injury) or traction injuries to the axillary nerve (most commonly in
two-part greater tuberosity anterior fracture-dislocations).24,64,280,298,299
Careful assessment of the plexus and its branches must be undertaken
after any proximal humeral fracture. Electromyography and nerve
conduction studies should be requested to delineate the extent of nerve
injury and the prospects for recovery.
elicit after an acute proximal humeral fracture. Cuff dysfunction is
inevitable if there is a tuberosity fracture, because the attached cuff
is defunctioned. The integrity of the cuff can only be assessed
clinically when the fracture heals, allowing the tendon to function
again. However, isolated rotator cuff avulsions and ruptures are
commonly encountered,89 even with
fractures that do not involve the tuberosities. Imaging of the rotator
cuff should be obtained at an early stage if signs persist, using
ultrasound or preferably MRI. It is important to appreciate that many
tears may predate the injury or be “acute-on-chronic” tears that have
been aggravated by the injury.
injuries, it is important to assess for evidence of other systems
injuries using Advanced Trauma Life Support (ATLS) secondary survey
guidelines, particularly in the presence of a high-energy injury.
Ipsilateral chest injuries (rib fractures, hemothorax, or pneumothorax)
and cervical spine injuries are commonly associated with shoulder
injuries in high-energy trauma. Intrathoracic and retroperitoneal
fracture-dislocation of the humeral head have also been reported but
are extremely rare.281,310
fractures of the hip after low-energy injury. Simultaneous fractures of
the wrist, forearm, or elbow are also relatively common and may be
masked by the pain and swelling in the shoulder. Conversely, it is
important to exclude a shoulder injury in any patient with shoulder
pain associated with a more distal extremity fracture. Assessment may
be particularly difficult in patients who have an ipsilateral humeral
shaft fracture. Good-quality orthogonal radiographs, centered on the
shoulder must always be obtained to exclude an associated fracture or
dislocation.157,216
health, fitness for anesthesia, and ability to cooperate with prolonged
rehabilitation should be made. As with any other osteoporotic fracture,
an assessment of the severity of osteoporosis should be undertaken.
Secondary preventative treatment should be considered, aimed at
reducing the risk of further osteoporosis-related falls and fractures.
Dietary supplementation with calcium and vitamin D may also be of
benefit in improving the rate of fracture healing.69
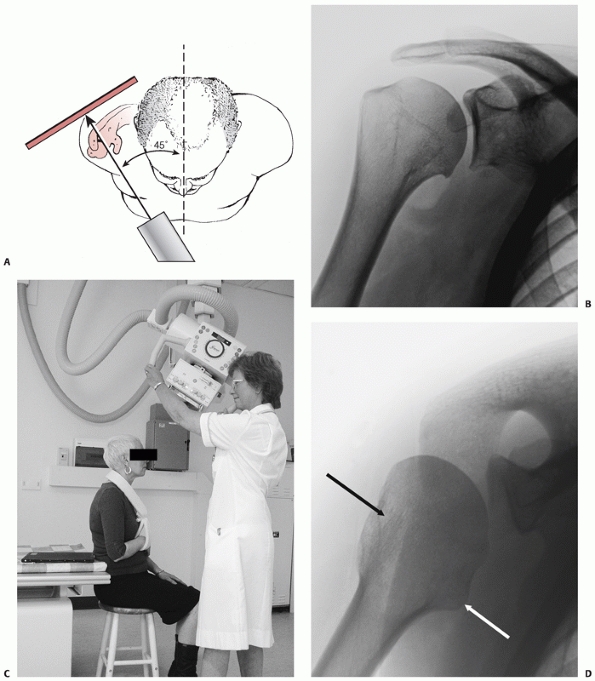
consists of anteroposterior and lateral radiographs, together with an
axillary view. The latter may be uncomfortable to obtain after injury,
and the Velpeau view79 or modified axillary view302 are considered acceptable substitutes, because these can be taken without removing the painful fractured arm from the sling (Fig. 35-6).
Anteroposterior radiographs taken in the standard anatomic planes are
often unsatisfactory, because the glenoid, coracoid, and acromion may
overlap the proximal humeral fracture.
The
best depiction of the fracture is therefore obtained if orthogonal
views are taken in the plane of the glenoid. Since the scapula is
protracted on the chest wall, the anteroposterior view is usually
obtained by tilting the x-ray beam approximately 30 degrees medial to
the normal anatomic plane (see Fig. 35-2).
 |
|
FIGURE 35-6 A. The true anteroposterior view of glenohumeral joint requires the beam to be angled 45 degrees from the sagittal plane. B.
The true anteroposterior view in the plane of the scapula is orthogonal to the face of the glenoid to show the maximum amount of the humeral head articular surface possible. C. The modified axial view is obtained with the arm resting in a sling and the x-ray gantry tilted 45 degrees in the sagittal plane. D. The view clearly shows the outline of the greater tuberosity (black arrow) and lesser tuberosity (white arrow) and confirms congruent reduction of the humeral head. |
and displacement of the shaft with respect to the head in a proximal
humeral fracture is typically anteromedial.207
This deformity is therefore seldom adequately visualized on standard
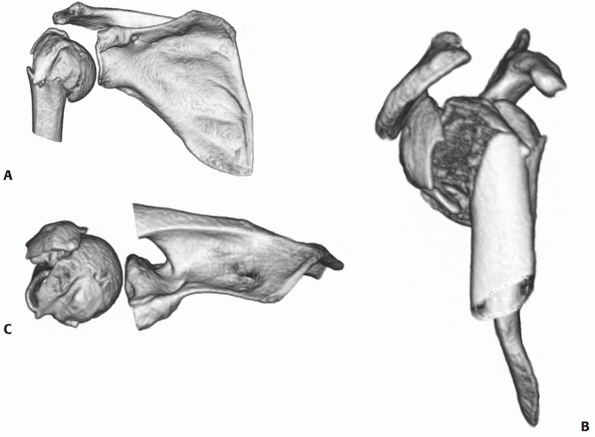
trauma series radiographs. Computed tomography (CT) scanning is being
increasingly used to provide further detail of the complex fracture
anatomy (Fig. 35-7).73 Most modern spiral scanners have
the software facility for three-dimensional reformatting of the images,137
to provide a better representation of the complex anatomy of these
injuries. These images may also aid in the detection of articular
surface injuries, which are often not visualized on conventional
radiographs.73
 |
|
FIGURE 35-7 The standard three-dimensional computed tomography reconstruction views of a fracture: (A) anterior view in the scapular plane (with the coracoid truncated), (B) the lateral view, and (C) the superior view (with the acromion truncated).
|
as it provides less detail about the bone architecture than CT. In
addition, the deformity produced by the fracture may hinder
interpretation of the soft tissue anatomy of the injury. MRI may be
useful in imaging the integrity of the rotator cuff in patients who
have complications after their initial treatment and require later
reconstructive surgery.
serve as a guide to treatment and predict outcome. Although
classification of proximal humeral fractures has tended to focus on the
fracture configuration, it is important to appreciate that there are
important patient- and treatment-related factors that determine the
outcome after these injuries.
that evaluated displacement and the presence of an associated shoulder
dislocation had been described before the 1970s.150
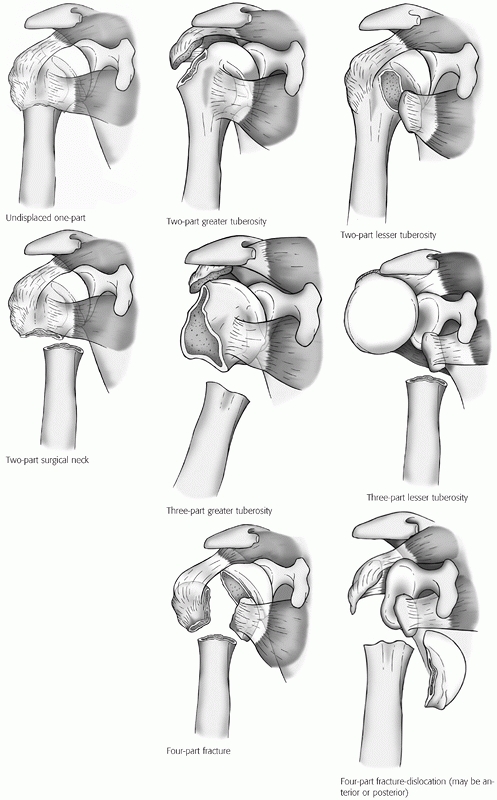
Codman recognized that the proximal humerus tends to fracture along its
physeal lines of fusion into four principal fragments (the two
tuberosities, the humeral head, and the shaft).48 Neer subsequently refined these definitions to produce the classification that remains most widely used today.207,208
Each of the four fragments are considered as unique parts only if they
are separated by more than 1 cm or angulated by more than 45 degrees to
one another (Fig. 35-8). These parameters were
selected somewhat arbitrarily, and reproducible and reliable
measurement of the degree of displacement and angulation is difficult
using conventional radiographs.19,276,277
Displaced fractures are classified according to the number of displaced
fragments, regardless of the number of secondary fracture lines, into two-, three-, or four-part configuration.
Fracture-dislocations are also classified according to the direction of
displacement of the humeral head (anterior or posterior), as well as
according to the number of fracture fragments. Using these criteria,
Neer suggested that 85% of fractures are minimally displaced, although
more recent studies suggest a prevalence of closer to 50%.57,129,316
but none has proved as robust over time or gained the general
acceptance of the Neer classification. It has been criticized for
lacking reproducibility,19,276,277
and since it is an anatomic classification, it does not directly serve
as a guide to treatment or directly predict outcome. The complex
three-dimensional deformity of these fractures can only be adequately
appraised at the time of surgery291 or using CT with three-dimensional reconstructions.73,266
survived intact for the past 40 years, although at the time when the
classification was first produced there was a limited range of
treatment options available to treat proximal humeral fractures. The
recent advances in the technology available to assess and treat these
fractures, together with an improved appreciation of the factors
associated with a more benign prognosis after reconstruction, have led
to a refined description of some fracture
configurations.
Some of these configurations did not feature in the original
descriptive classification but nevertheless provide useful additional
prognostic information. Until a more comprehensive classification is
produced, which guides treatment and predicts outcome, it is best to
view these as “descriptive modifiers” of the original Neer
classification, which may alter the treatment and outcome for some of
the fracture subtypes. For completeness, the AO/OTA classification is
given together with the modified Neer classification in the following
sections.
 |
|
FIGURE 35-8
The main subgroups of Neer classification of proximal humerus fractures. (Modified from Neer CS. Displaced proximal humeral fractures: I. Classification and evaluation. J Bone Joint Surg Am 1970;52A:1077-1089, with permission.) The rarer articular surface fractures (head-splitting or impression fractures) are not depicted here, but are described freely in the text and depicted in Figures 35.12, 35.16, and 35.17. |
|
TABLE 35-1 The OTA Classification of Proximal Humeral Fractures
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
fractures are undisplaced, when Neer’s criteria for displacement are
strictly applied57,86,156 (Table 35-2).
Such estimations can only be approximate because of the difficulties in
estimating angulation and displacement on conventional radiographs.
Minor fractures may not reach the attention of orthopaedic services or
may be diagnosed late.228,237,317 Undisplaced fractures tend to occur in a slightly younger, fitter group of patients,57,86,156,221,222
possibly because they have better proximal humeral bone stock and a
stronger and thicker periosteal sleeve of tissue preventing fracture
displacement. Despite their prevalence, these injuries tend to receive
less attention in the literature, because most are treated
nonoperatively and have a good prognosis.
|
TABLE 35-2 The Relative Prevalence of Proximal Humeral Fractures According to the Neer Classification
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
either the anatomic or surgical neck of the humerus and in one or both
tuberosities (Fig. 35-9). Although secondary
displacement is possible, especially if the head is not impacted on the
shaft fragment, this is relatively unusual. Inferior subluxation of the
humeral head may occasionally occur through hemarthrosis, muscle atony,
or capsular injury. This almost always spontaneously resolves with
nonoperative treatment over a period of a few weeks. Minimally
displaced greater tuberosity fractures can be associated with rotator
cuff tears, and this may be the source of persistent disability after
fracture.149 Arthroscopic debridement and repair may be successful in these patients.149
literature, but they are common, accounting for approximately 10% of
all proximal humeral fractures57 (Table 35-2).
The recovery from this injury may be protracted because of the
associated defunctioning or impingement of the rotator cuff, which they
may produce. These injuries have a spectrum of severity: approximately
half are isolated fractures, and the remainder are associated with
glenohumeral dislocations and associated soft tissue injury, most
commonly an associated nerve injury. Those associated with a
glenohumeral dislocation tend to occur in the middle-aged and elderly,
as distinct from isolated dislocations, which tend to occur in younger
individuals. The “terrible triad” of the shoulder occurs when the
tuberosity avulsion fracture is associated with anterior dislocation of
the shoulder and an associated nerve or plexus injury.108
This injury pattern is relatively common, although the exact prevalence
has not been defined. It represents the most severe form of injury,
from which functional recovery is often incomplete.
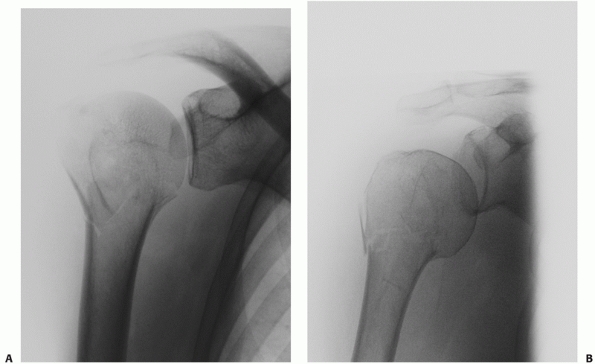 |
|
FIGURE 35-9 A,B.
Undisplaced and stable fracture configurations are best treated nonoperatively in most elderly patients, as shown in these two examples. |
However, isolated greater tuberosity fractures are seldom associated
with glenohumeral instability, possibly because there is an inferior
capsular rupture rather than the typical anteroinferior labral
detachment (Bankart lesion), which more commonly affects younger
patients.248,249,261
An injury produced by axial loading may produce a primary anatomic neck
fracture via the same mechanism as for an impacted valgus fracture. The
displacement of this fracture may be slight but sufficient to cause a
secondary greater tuberosity fracture. The greater tuberosity fracture
is often the most striking feature on the initial trauma series
radiographs, and the anatomic neck fracture may not be visible if the
correct anteroposterior views of the shoulder are not taken (Fig. 35-10). It is estimated that approximately 10% of isolated greater tuberosity fractures may have a concomitant
anatomic neck fracture. Alternatively a tuberosity fracture may be
produced by a traction injury, often during a glenohumeral dislocation.
This may either be from a bony avulsion of the rotator cuff, or through
propagation of an acute osteochondral fracture of the posterior humeral
head (Hill-Sachs lesion), as it engages on the anterior glenoid.8,73,107
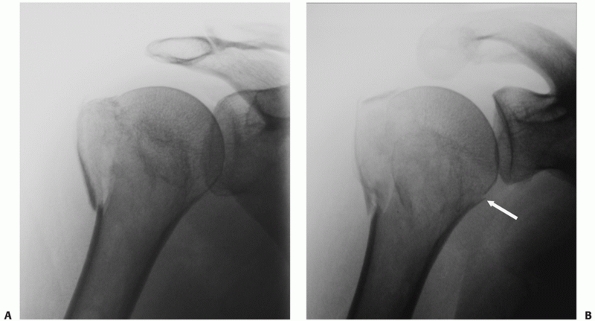 |
|
FIGURE 35-10 Many seemingly isolated greater tuberosity fractures (A) actually have undisplaced anatomic neck fracture lines (arrow), which are only seen when a correctly orientated anteroposterior view is taken (B).
|
quite widely, ranging from small, often multifragmentary injuries to
larger single-fragment fractures (Fig. 35-11).
The former should be regarded as a rotator cuff avulsion injury and has
a tendency to retract over time because of the unopposed pull of the
attached supraspinatus and infraspinatus tendons. The latter injury is
less prone to redisplacement. While Neer considered 1 cm of
displacement of any fracture fragment to be clinically significant,
recently some authors have suggested that 5 mm of displacement should
be considered a more appropriate threshold and indication for operative
treatment for these injuries, given the high risk of cuff dysfunction
and impingement even with this degree of displacement.31,81,224
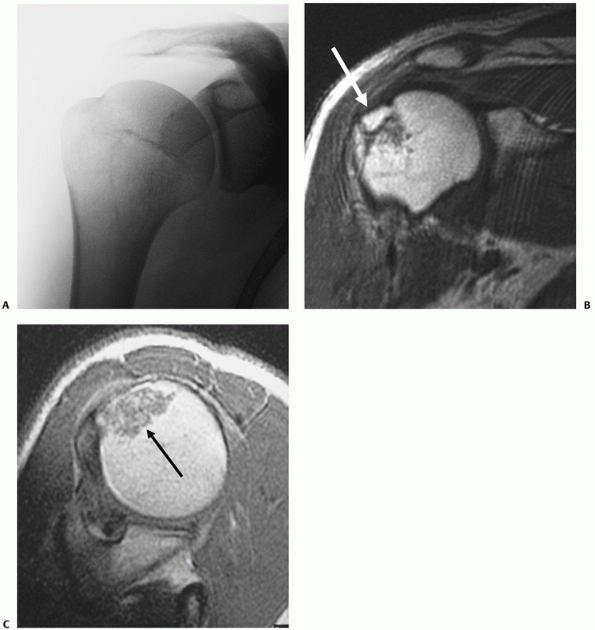 |
|
FIGURE 35-11 An undisplaced greater tuberosity fracture may not be visible on conventional radiography (A) and may only be seen on magnetic resonance imaging (B,C). (continues)
|
very rare injuries but are probably more common than undisplaced
(onepart) lesser tuberosity fractures, because of the tendency of the
attached subscapularis tendon to retract the fragment medially (Fig. 35-12).
They are atypical of other proximal humeral fractures, because they
tend to occur in middle-aged adults, with a slight male predominance,
and are usually produced by relatively high-energy injuries.72,177,218,257
bony avulsions of the subscapularis tendon, usually from a forced
external rotation injury.257 These fractures may also occur in association with posterior glenohumeral dislocations. In these
circumstances, the fracture is caused by propagation of the acute
osteochondral fracture of the anterior humeral head (reverse Hill-Sachs
defect), as it engages on the posterior glenoid.257
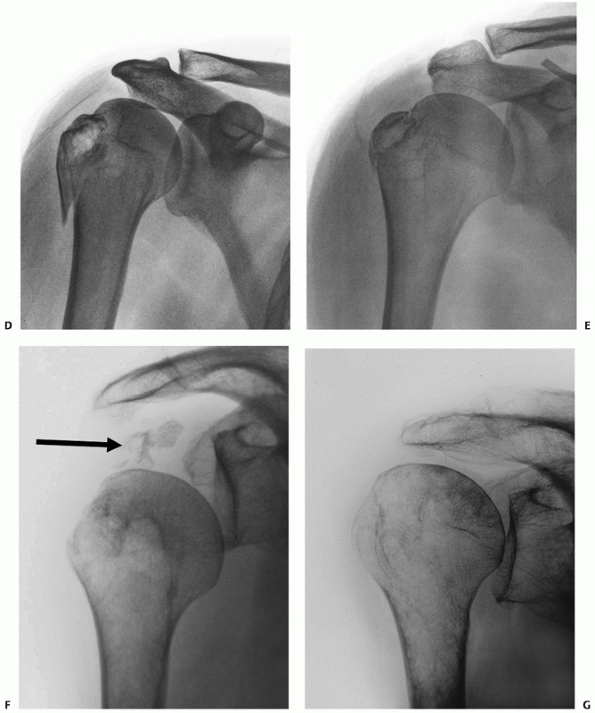 |
|
FIGURE 35-11 (continued) Greater tuberosity fractures vary widely in their size, ranging from larger fragments (D) to smaller avulsion-type injuries (E).
These may displace in the same manner as a rotator cuff tear, with retraction of the tendon leading to progressive displacement of the bone fragment (arrow) (F). Untreated, this may lead to a rotator cuff-deficient shoulder with a high-riding humeral head and rotator cuff arthropathy (G). |
humeral fractures and they tend to occur in a slightly older group of
individuals than the remainder of the proximal humeral fracture
population (Table 35-1). They are
extra-articular fractures, although undisplaced secondary tuberosity
fracture lines may be seen. Since the soft tissue attachments and blood
supply to the humeral head and tuberosities are preserved, there is a
low risk of osteonecrosis.
The angulated fracture may either be in neutral alignment, or such that
the head and attached tuberosities are tilted into varus
or valgus.73
The shaft is usually impacted onto the humeral head, and the direction
and extent of the head displacement are determined by the orientation
of the shaft as it is driven up within the metaphysis and the manner in
which the metaphyseal bone fails at the time of fracture. Because of
their impaction, these fractures have a low risk of complete
displacement and nonunion. However, the impacted varus fracture is
unusual in that the degree of angulation of the head on the shaft may
increase with nonoperative treatment.59
 |
|
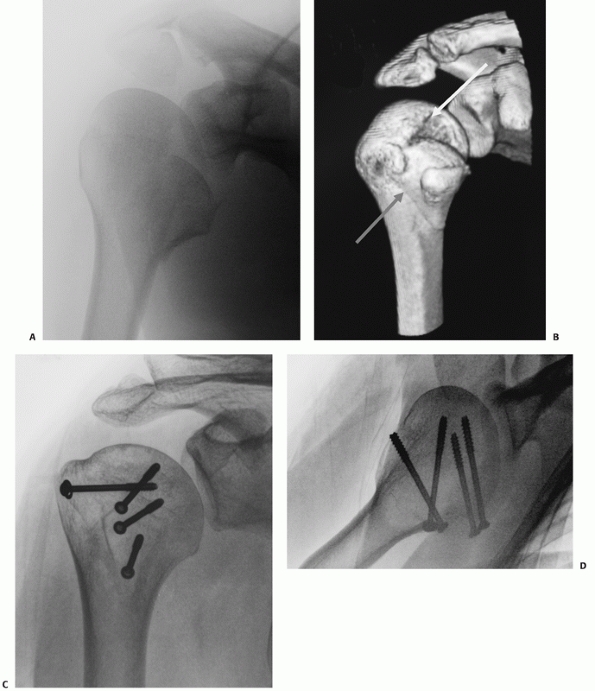
FIGURE 35-12 Isolated lesser tuberosity fractures are uncommon (A).
Their configuration is best appreciated using three-dimensional computed tomography, which shows the extension onto the humeral articular surface (white arrow) and involvement of the bicipital groove (gray arrow). B. Displaced fractures should be treated by either interosseous suturing or screw fixation (C,D). |
produced either by translation and separation of the shaft on the head
or through extensive comminution of the metaphyseal area, leading to
loss of cortical continuity.207 Displacement may be incomplete, with some residual cortical contact, or the head
may be completely disconnected for the shaft.58,73
The shaft tends to be pulled anteromedially, through the pull of the
pectoralis major. With complete displacement, the head either adopts a
neutral position or may progressively tilt into a varus position due
the pull of the attached rotator cuff muscles. Spontaneous reduction of
these fractures seldom occurs, and they have a higher risk of nonunion
after nonoperative treatment.58,60
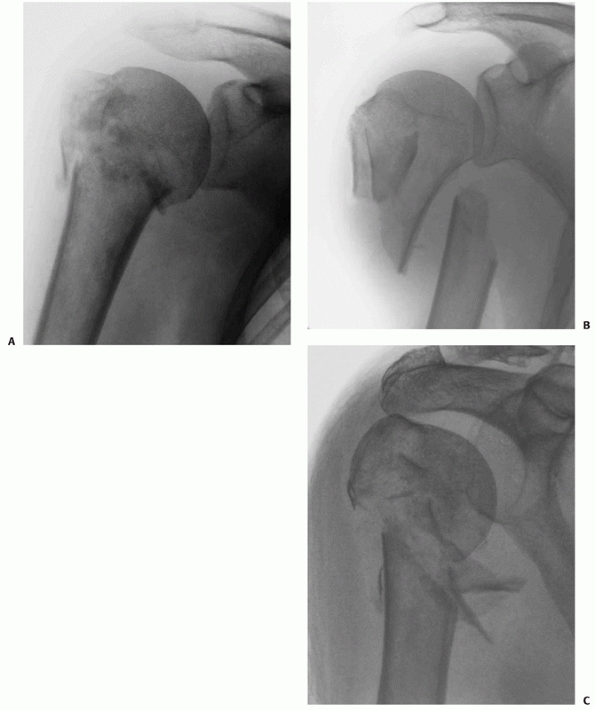 |
|
FIGURE 35-13 Displaced two-part surgical neck fractures can be either impacted (A), translated (B), or comminuted (C).
|
Any experience of their treatment and prognosis is likely to be
anecdotal, although Neer described these fractures as having a high
rate of osteonecrosis.207,208
This fracture configuration occurs most frequently in association with
posterior dislocation of the fractured humeral head (see later).
from this group of multifragmentary injuries, which account for
approximately 10% of proximal humeral fractures (Table 35-2).
On initial inspection, they appear to be a diverse group, in which the
extent of comminution and displacement of the three or four fracture
fragments varies markedly. However, most of the variation in the
fracture lines and their displacement can be explained by an
understanding of the deforming forces that
produce the constant feature of a primary fracture of the anatomic neck of the humerus.73
Tuberosity fractures are thought to be a secondary phenomenon, which
are caused by displacement of the humeral head relative to the shaft.
The fracture configuration is determined by the direction and severity
of displacement of the humeral head, and the deforming forces produced
by the residual soft tissue attachments of each fracture fragment.
risk of osteonecrosis after nonoperative treatment or head-conserving
reconstruction, because of the disruption of the soft tissue
attachments to the humeral head. It is now recognized that conventional
radiographs overestimate the degree of soft tissue and tuberosity
detachment from the head, and the risk of humeral head ischemia and
later osteonecrosis. Seeking additional descriptive evaluation of the
fracture is therefore to be encouraged. This should include, in
addition to evaluation of the number of fracture parts, a detailed
assessment of the configuration and orientation of the fracture parts
and an assessment of the potential viability of the humeral head and
the extent of articular surface involvement. Definitive assessment of
these parameters can only be made at the time of surgery, although
preoperative three-dimensional CT greatly facilitates evaluation and
planning the reconstruction. These factors are discussed in more detail
next.
 |
|
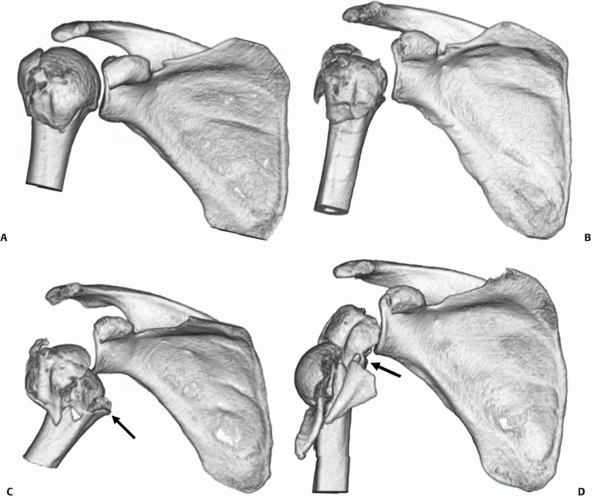
FIGURE 35-14
Three- and four-part impacted valgus fractures show considerable variation radiologically and on three-dimensional computed tomography reconstructions (as shown), ranging from fractures that are minimally displaced (A), through more severe valgus angulation (B) to displacement with lateral translation of the head, where the medial soft tissue hinge is disrupted (arrow) and the risk of osteonecrosis is higher (C,D). |
two-part surgical neck fractures, in three- and four-part fractures,
the humeral head may be either impacted on the shaft, or unimpacted,
when it may translate or separate from the shaft. As with two-part
surgical neck fractures, the humeral head may have a neutral, valgus,
or varus angulation.
fracture configuration, the humeral head occupies a neutral position in
the coronal plane on anteroposterior view. It may be internally rotated
in the horizontal plane, if there is a three-part greater tuberosity
fracture, because of the pull of the subscapularis on the attached
lesser tuberosity, whereas in four-part fractures, the head tends to
adopt a neutral position.
In this fracture configuration, the fractured humeral head faces
superiorly (in valgus), with the tuberosities splayed on either side of
it (Fig. 35-14). Its anatomic
features were described subsequent to the Neer classification,207,289 and Jakob was the first to recognize its benign prognosis compared with other multipart fractures.134 It is one of the more common patterns of fracture56,57 and represents a spectrum of injury, with variation in the degree of humeral head angulation and displacement.56,65
Recognition of this variation is important, because it is now realized
that treatment aimed at preserving the native humeral head may not
always produce satisfactory results.
may be slight and these fractures merge with the previously described
two-part greater tuberosity fracture configuration. If the head is
pushed farther into the metaphysis, there is a greater degree of valgus
impaction.73 The intact medial
periosteal hinge and capsule between the head and the calcar is the
axis around which displacement occurs and may act as a source of
perfusion to the humeral head.
with the articular surface facing directly superiorly, the sharp medial
calcar is exposed and the medial hinge of periosteum begins to tear as
it is stretched over the unyielding edge of the calcar. This causes
lateral translation of the head relative to the calcar, resulting in
further propagation of the tear in the periosteal hinge.
tolerate before it becomes completely denuded of soft tissue
attachments and at higher risk of osteonecrosis of the head is unclear.
Cadaver studies suggest that the medial periosteal hinge completely
ruptures at between 6 to 11 mm of lateral head displacement,7,113,240,241 although it has been suggested that as little as 5 mm of displacement may be sufficient to cause complete disruption272 (Fig. 35-14).
recognized, in which the humeral head is invariably devoid of soft
tissue attachments and ischemic. The humeral head may remain attached
to the shaft and dislocate anteroinferiorly. This injury pattern is
discussed together with other fracture-dislocations below (see Type II
anterior fracture-dislocations). Alternatively, the lateralization of
the humeral head caused by the medial displacement of the shaft may
progress such that the head becomes completely disengaged from the
shaft. This pattern of injury may occur de novo or be produced
iatrogenically through an attempted closed reduction of a lower grade
fracture.77,121,252
four-part fractures in which the humeral head is tilted into varus have
received less attention than valgus fractures but nevertheless
constitute a substantial proportion of these injuries (Fig. 35-15).
They are thought to represent a more severe end of the spectrum of
two-part surgical neck impacted varus fractures, in which undisplaced
fractures in the tuberosities open up and displace.73
As with two-part surgical neck varus fractures, the humeral head has a
tendency to tilt into a progressively greater degree of varus with
nonoperative treatment.
classic description of the two tuberosity fracture fragments has
undergone some redefinition,207
since it is now appreciated that most tuberosity fractures occur
secondary to the displacement of the head fragment. Their degree of
spatial displacement is initially minimal, relative to their normal
anatomic position. With nonoperative treatment, progressive
displacement may occur because of the unopposed pull of the rotator
cuff muscles.
 |
|
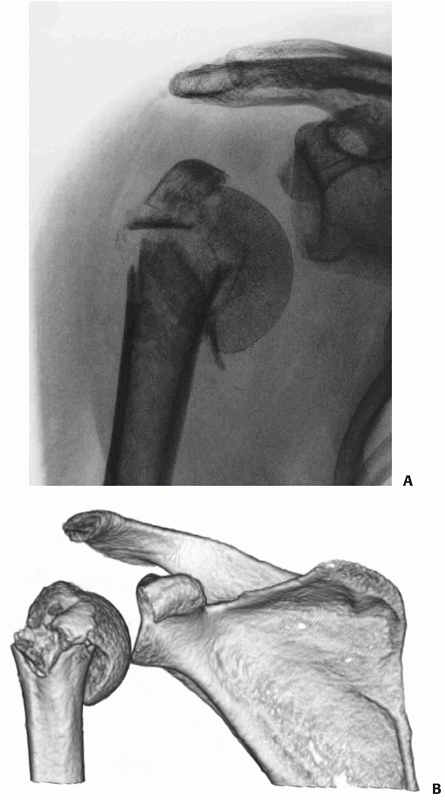
FIGURE 35-15
Three- and four-part varus fractures are less common than valgus injuries, and radiologically give rise to inferior subluxation of the humeral head on conventional radiography (A) and on three-dimensional reconstructions of computerised tomograms (B). |
extremely uncommon injuries, accounting for only 0.3% of all proximal
humeral fractures,57 and most of the remaining 10% of three-part fractures therefore involve the greater tuberosity (Table 35-1).
The bicipital groove does not form a plane of cleavage between the
tuberosities, as conceived in the original concept of the injury.49,207
Instead, the characteristic initial tuberosity fracture line is located
posterior to the hard cortical bone of the groove, in the softer bone
of the anterior portion of the greater tuberosity, just lateral to the
supraspinatus facet.73 A separated
greater tuberosity fragment may itself be comminuted and tends to
retract posterosuperomedially because of the pull of the infraspinatus
and teres minor tendons (Fig. 35-16).
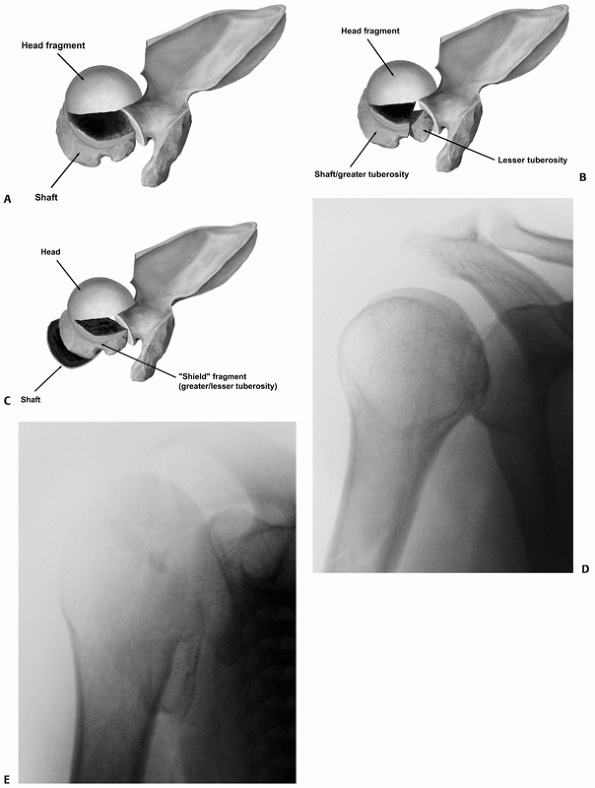
remaining composite tuberosity fragment, which comprises the anterior
portion of the greater tuberosity, the bicipital groove, the lesser
tuberosity, and often a portion of the adjacent articular surface (Fig. 35-16).
This composite “shield” fragment may either be intact, comminuted with
minimal separation of its component parts, or comminuted and separated
(“the shattered shield”). Most four-part fractures, as conceived by
Neer,207 fall into the latter group and account for a small minority of all these fractures (Table 35-2). Although other fracture configurations have been described,123,195,291 in practice these other types of two- and three-part fractures are extremely rare.
 |
|
FIGURE 35-16
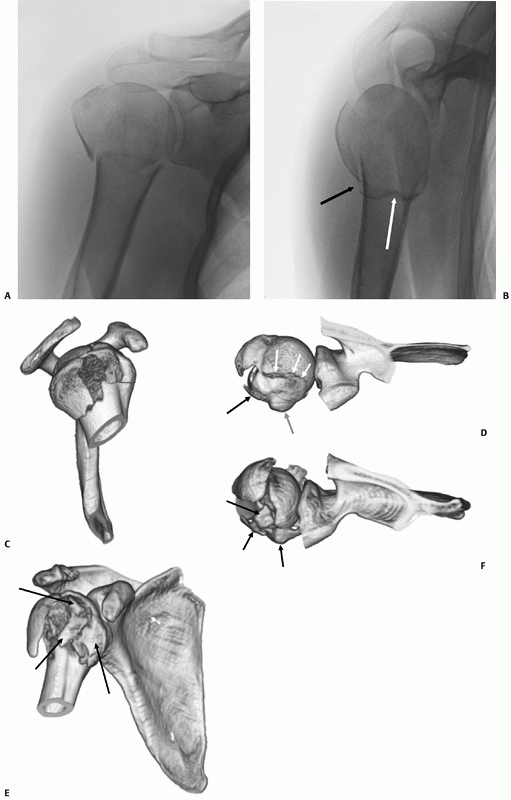
The tuberosity fracture configuration in threeand four-part fractures follows three common configurations on anteroposterior, modified axial, and three-dimensional computed tomography reconstructions. In a three-part greater tuberosity fracture, the primary fracture line (black arrow) is always located posterior to the bicipital groove (white arrow) on the modified axial view (A,B). In a four-part fracture, there is in addition a composite shield fragment consisting of the lesser tuberosity, the bicipital groove (gray arrow), the anterior portion of the greater tuberosity (black arrow), and frequently an adjacent marginal portion of the articular surface (white arrows), as shown on these three-dimensional computed tomography reconstructions (C,D). The shield fragment may be comminuted or “shattered,” leading to a more characteristic four-part fracture configuration. In this case, the three-dimensional reconstructions show at least three separate fractured components of the shield (arrow) (E,F). |
configuration is important since a large shield fragment may produce an
obstacle to gaining access to the displaced humeral head fragment, if
operative treatment is selected. The predictable and progressive
displacement of unhealed tuberosity fragments may lead to later
problems from rotator cuff impingement and dysfunction. Humeral Head
Viability and Risk of Osteonecrosis. The risk of osteonecrosis of the
humeral head after three- and four-part fractures prompted an attempt
to identify factors that are predictive of this complication.
Back-bleeding after bore-hole insertion in the cancellous bone of the
humeral head and Doppler flowmetry to detect humeral head blood flow
have been used to quantify the degree of humeral head ischemia at the
time of surgery.123 The presence of
a longer posteromedial metaphyseal spike of bone attached to the
humeral head (longer than 8 mm) may be associated with a high rate of
intraoperative head perfusion, presumably through retained blood flow
through capsular attachments in this area. In addition, preservation of
a medial hinge in a valgus fracture, without lateralization of the
head, is also associated with a greater likelihood of humeral head
perfusion.123 However, there is a
poor correlation of the presence of intraoperative head blood flow with
the later development of osteonecrosis.14
Some humeral heads that are perfused at surgery later develop
osteonecrosis, whereas some ischemic heads did not develop
osteonecrosis after internal fixation. At present, there is no reliable
method to accurately predict the development of osteonecrosis of the
humeral head after fracture.
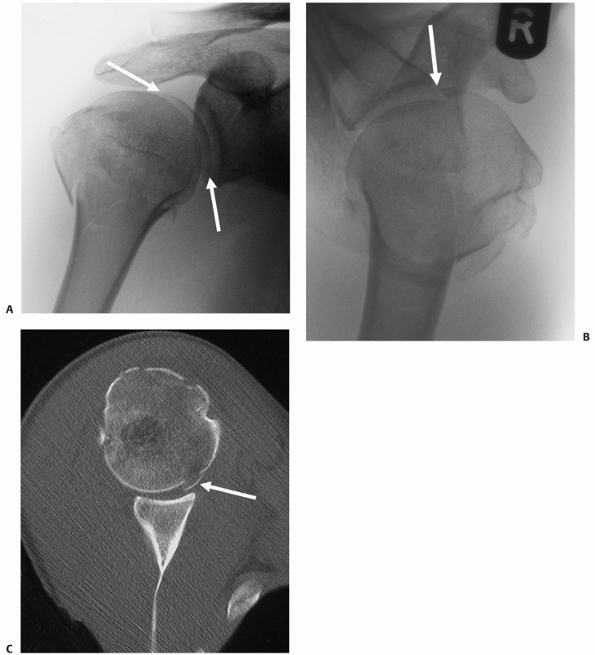
description, articular surface fractures were regarded as a separate
subgroup of fracture-dislocations. It is now recognized from CT studies
that these injuries are more common than was previously appreciated.
Fractures that involve the articular surface can occur through a
variety of mechanisms: In the most severe and rare form, the larger
surface area of the humeral head may be cleaved as it impacts against
the narrow “anvil” of the glenoid into two or more large fragments in
the true “head-splitting” fracture.
intra-articular fractures to carry peripheral portions of the articular
surface with them as “marginal” fragments. This type of articular
injury is particularly associated with larger shield fractures, which
often have a portion of the adjacent superolateral humeral articular
surface attached to them (Fig. 35-17). These
fragments may be difficult to see on plain radiographs, although a
“double shadow” of the articular surface is usually pathognomic (Fig. 35-17).
Failure to anatomically reduce these articular fragments may compromise
the reconstruction and lead to early secondary osteoarthrosis.
through direct injury when the humeral head articular surface impacts
on the anterior or posterior glenoid rim during a posterior or anterior
glenohumeral dislocation, respectively. If the acute osteochondral
fracture of humeral head (Hill-Sachs lesion or reverse Hill-Sachs
lesion) propagates, this may produce a Type I anterior-fracture
dislocation or a posterior fracture-dislocation. These are discussed in
more detail in the next section.
and anterior are much more common than posterior fracture-dislocations.
The terminology that has been used to describe these injuries is
confusing—in continental Europe, the term “dislocation” has often been
used synonymously with “displacement.”119,145,211
To avoid ambiguity, the term “fracture-dislocation” is best reserved
for injuries in which there is complete dissociation of the fractured
humeral head from the glenoid.
treatment of complex multipart proximal humeral fractures, and they are
traditionally regarded as representing the more severe end of the
spectrum of multipart fractures, with a higher risk of osteonecrosis.
However, there is evidence to suggest that the mechanism of injury and
prognosis may be different for some anterior fracture-dislocations and
the majority of posterior fracture-dislocations.73,207,209,245,252
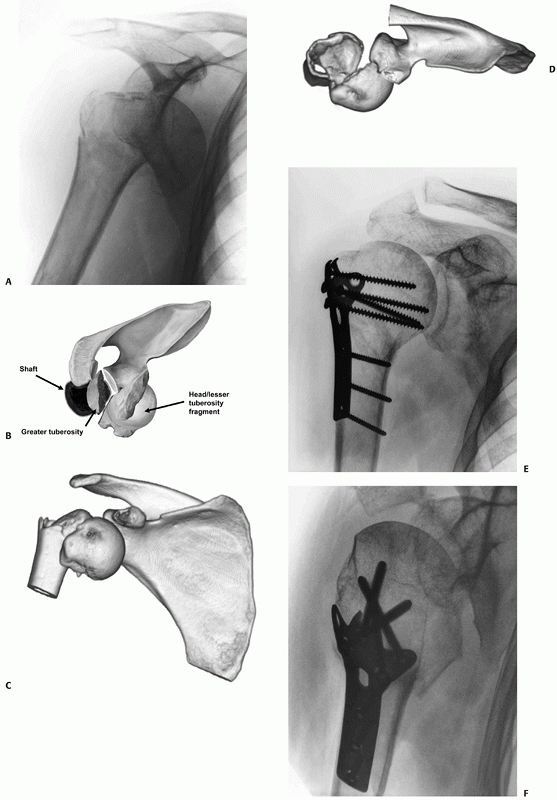
recognition that some anterior fracture-dislocations have a better
prognosis following open reduction and internal fixation (ORIF) has led
to classification into two subtypes, as follows.
The humeral head commonly retains capsular attachments through both an
intact periosteal sleeve around the lesser tuberosity in a three-part
fracture-dislocation and a retained extracapsular posteromedial bone
spike attached to the head. The pathologic features of the injury
resemble those of a first-time anterior dislocation of the glenohumeral
joint,248 because there is a soft
tissue or bony avulsion of the anteroinferior capsulolabral complex (a
soft tissue or bony Bankart lesion) and an acute osteochondral fracture
of the posterior humeral head (Hill-Sachs lesion) caused by its
impaction on the anterior glenoid rim. The fracture is produced by
propagation of the Hill-Sachs lesion, through the anatomic neck of the
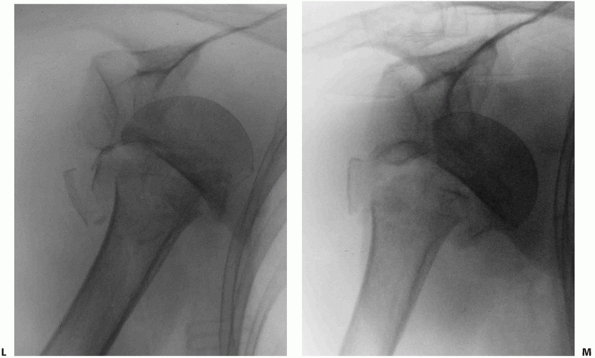
humerus, and therefore occurs after the head has dislocated252 (Fig. 35-18).
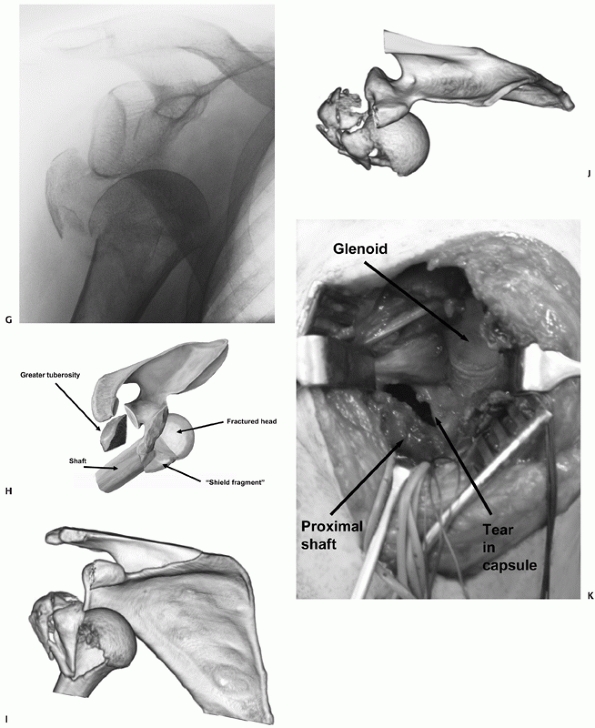
The Type II injury is more common, and mostly occurs in older females,
who sustain their injuries in low-energy trauma. Radiologically, the
fracture resembles a three- or four-part valgus fracture, but with the
humeral head dislocated anteroinferiorly, and not engaged on the
glenoid. The humeral head fractures in a valgus position and the
exposed sharp medial calcar tears through the inferomedial capsule in
the region of the axillary recess. The shaft displaces through this
capsular rent, carrying the impacted humeral head with it
(Fig. 35-18).
Any remaining capsular attachments to the head are torn during the
dislocation. The importance of the recognition of this injury is that
although the fracture configuration is similar to the valgus fracture
(which typically has a benign prognosis), the dislocated humeral head
is devoid of capsular attachments and blood supply and is therefore at
much higher risk of osteonecrosis. The results of a head-salvaging
reconstruction in this injury type are therefore poor.
 |
|
FIGURE 35-17 A. “Double-shadow” on the anteroposterior radiograph (arrow) is pathognomic of a head-split fracture. The split is also seen on the modified axial view (B) but the extent of head involvement is best assessed using a computed tomography scan (C). The white arrows show the head-split.
|
undisplaced anatomic neck fractures are difficult to detect on standard
radiographs, and these injuries are commonly initially mistaken for
two-part greater tuberosity fracture-dislocations.121,252
Secondary displacement of the head may be produced if an attempt is
made to obtain a closed relocation by manipulation. CT should be
performed for all anterior shoulder dislocations with a greater
tuberosity fracture, where an undisplaced anatomic neck fracture is
suspected on the trauma series radiographs. Two-, Three-, and Four-Part
Posterior Fracture-Dislocations. Posterior fracture-dislocations are a
rare but important group of proximal humeral fractures, which occur in
a relatively young, middle-aged group of predominantly male patients.
This injury may be bilateral, when it is usually produced by a seizure,
caused by either epilepsy, alcohol or drug withdrawal, or hypoglycemia.245 Unilateral injuries typically occur from falls from height or road traffic accidents.
fracture-dislocation: The fracture of the anatomic neck propagates from
the area of an osteochondral fracture of the anterior humeral head
(reverse Hill-Sachs lesion), as it engages on the posterior glenoid
rim. The posterior dislocation of the humeral head avulses the
posteroinferior capsulolabral soft tissue sleeve and produces a reverse
Bankart lesion (capsulolabral avulsion of the posteroinferior glenoid
rim) in all cases. However, the capsule and periosteal sleeve are in
continuity, and the anatomic neck fracture
“hinges”
on these structures. Three fracture subtypes are determined by the type
and extent of “secondary” fractures lines in the tuberosities245 (Fig. 35-19). Their recognition is important, because they serve as a guide to the technique of internal fixation that should be used.
 |
|
FIGURE 35-18
In Type I anterior fracture-dislocations, the humeral head is engaged on the anterior glenoid rim, as shown on the anteroposterior radiograph (A), the schematic diagram (B), and anteroposterior and superior three-dimensional computed tomography reconstructions (C,D). Internal fixation of these fractures is associated with a low risk of osteonecrosis (E,F). (continues) |
 |
|
FIGURE 35-18 (continued)
In Type II anterior fracture-dislocations, the humeral head is not engaged on the anterior glenoid rim, as shown on the anteroposterior radiograph (G), the schematic diagram (H), and anteroposterior and superior three-dimensional computed tomography reconstructions (I,J). The humeral head dislocates through a rent in the inferior capsule (K). (continues) |
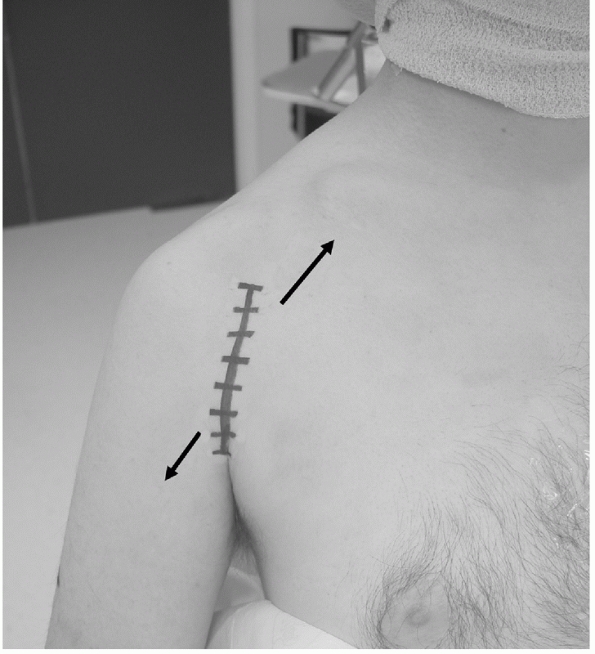
this “utility” approach,106,159,255,305
which is extensile and follows normal anatomic planes. The cephalic
vein should be identified and protected, and it is customary to reflect
the vein laterally, as there are less feeding vessels to this side to
be ligated. The traditional skin incision, which follows the surface
markings of the deltopectoral interval, is now often replaced by a more
cosmetic skin incision, which is in the line of the anterior axillary
skin crease (along the line of the bra-strap in women). With more
extensive elevation of the soft tissue flaps in the proximal and distal
portions of this incision, similar access can be gained to that
obtained with the traditional incision (Fig. 35-20).
The approach is extensile (Henry approach) and can be continued
distally as an anterolateral approach if there is a diaphyseal
extension of the fracture. This skirts anterior to the deltoid
insertion and extends as an anterolateral approach between the
brachialis and triceps. In this situation, the radial nerve must be
exposed distally, as it emerges between the brachialis muscle and
brachioradialis muscles.
 |
|
FIGURE 35-18 (continued)
It is usually devoid of soft tissue attachments and at higher risk of osteonecrosis. Iatrogenic displacement of the initially impacted shaft from the head may occur during attempted relocation of the shoulder, as seen on these premanipulation and postmanipulation radiographs that were taken in the emergency department (L,M). |
of the shoulder but only limited access to its posterolateral aspect.
Visualization and manipulation of a large retracted greater tuberosity
fragment may therefore be difficult in muscular individuals.
However, this approach provides good visualization of the
posterolateral aspect of the shoulder without the requirement for
extensive soft tissue dissection or forcible retraction. Although the
approach is widely used in shoulder arthroplasty surgery,51,178,191 in posttraumatic reconstruction it has been mainly used to treat greater tuberosity fractures.81
fracture surgery led to the development of novel extended
deltoid-splitting approaches. These identify and preserve the anterior
terminal branches of the axillary nerve, as they traverse the deep
surface of the deltoid muscle.153
The skin incision may be fashioned longitudinally as an extension of a
traditional deltoid-splitting incision, in line with the fibers of the
middle third of the deltoid.92,93,96 However, the author prefers to use a shoulder strap incision, with its apex centered over the tip of the acromion,146,244,245,251,253,254 because it follows the relaxed skin tension lines around the shoulder girdle32
and therefore tends to heal more cosmetically. It also has the
advantage that a simultaneous deltopectoral exposure may also be
performed at its lower anterior extension, if required.252
An extended longitudinal incision provides better exposure of the
diaphysis and should be used for fractures with extension into the
humeral shaft.
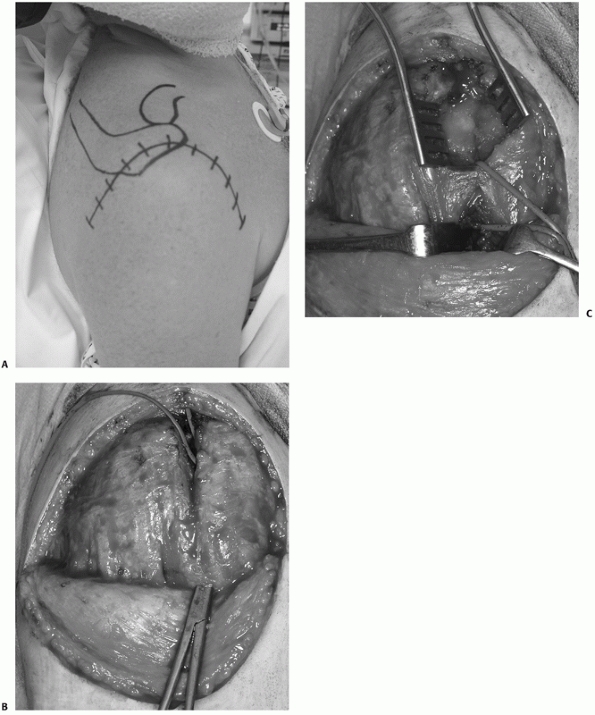
allows full exposure of the superior deltoid muscle. This is then split
in the line of its fibers at the junction of the anterior and middle
portions of the muscle. This site is relatively bloodless, as it is a
watershed area of the deltoid blood supply.111,130,204 This superior split produces the upper “window” of the approach and with further dissection allows visualization of the whole
anterolateral and posterolateral aspect of the proximal humerus (Fig. 35-21).
 |
|
FIGURE 35-19
Posterior fracture dislocations are of three subtypes, as shown in these schematic illustrations: In Type 1 injuries, there is a fracture of the anatomic neck alone (A); in Type II, there is in addition a lesser tuberosity fracture (B); and in Type III, there is in addition a “shield” fracture consisting of the lesser tuberosity, the bicipital groove, the anterior portion of the greater tuberosity, and frequently an adjacent marginal portion of the articular surface (C). These injuries may be missed on the standard anteroposterior radiograph (D) in the emergency department but are always identifiable on modified axial views (E). |
The deltoid split is then continued distal to the nerve, producing a
lower “window,” to view the lateral diaphysis. The approach is
versatile, allowing either ORIF or hemiarthroplasty to be performed
through the same incision. At the end of the procedure, meticulous
repair of the deltoid attachment to the acromion is important, to avoid
later detachment. Recent cadaveric studies have confirmed the safety of
deltoid-splitting surgical approaches that protect the anterior motor
branch of the nerve,92,93,96,146
although there remains theoretically an increased risk of injuring the
nerve and of later deltoid detachment from the acromion. The risk of
injuring these structures is also increased if secondary operative
procedures are required.
have been described for the exposure and treatment of
posterior-fracture dislocations. Open, combined deltopectoral and
deltoid-splitting
surgical
approaches have also been described for complex three- and four-part
fractures where adequate exposure cannot be gained through a single
incision.90,179 Minimally invasive and arthroscopic approaches are described in more detail in later sections.
 |
|
FIGURE 35-20
The deltopectoral approach should follow the anterior axillary crease for better cosmesis. Similar access to the traditional skin incision, which follows the surface markings of the interval, can be obtained by deep dissection of the skin flaps superolaterally anfd inferomedially (arrow). |
fractures is to promote complication-free healing to recreate a
pain-free, mobile, stable, and functional shoulder joint. In most
instances, this is best achieved with nonoperative treatment. While
functional normality may be reached in more innocuous injuries, with
more severe injuries this is seldom attainable, and the patient should
be appropriately counseled at an early stage. Three main groups of
factors determine outcome, and these should be taken into consideration
when deciding on treatment:
are treated nonoperatively, the choice of treatment in these injuries
is not markedly influenced by the patient’s physiologic status. The
minority of patients who require surgical treatment can be safely
treated operatively, with a low operative risk from anesthesia.
However, nonoperative treatment should be considered in frail, elderly
patients, with very limited functional expectations, or limited life
expectancy, irrespective of the radiologic severity of the fracture.
value of patient-related factors on outcome after proximal humeral
fracture following nonoperative treatment and hemiarthroplasty.25,56,58,59,255
The age of the patient is the most constant and well-defined
determinant of outcome after treatment. The adverse effect of advanced
age on clinical outcome is likely to be related to several factors
commonly seen in the elderly and frail patient, including cognitive
deficits,25 rotator cuff tears,200 osteoporosis,61 and difficulty with postoperative rehabilitation.25 Patients with a history of alcohol abuse have an increased risk of nonunion300 and are less likely to be compliant with postoperative rehabilitation regimens.25 Tobacco consumption also increases the risk of nonunion after proximal humerus fractures.259
Other commonly encountered medical comorbidities, which should be
considered as “modifiers” when deciding on treatment, are listed in Table 35-3.
The multitude of patient-related factors that affect outcome makes
their classification difficult, and currently there is no satisfactory
overall method of assessing this.
fracture classifications consider fracture displacement, the severity
of the soft tissue injury, or the presence of other skeletal injuries.
Their ability to guide treatment and predict outcome is therefore
limited.
fractures requires considerable surgical expertise, and the full
armamentarium of shoulder reconstructive implants, each with its
technical advantages and drawbacks (Table 35-4).
A technically poor, unstable reconstructive procedure will usually
produce a worse outcome than nonoperative treatment. Referral to a
center with a surgeon experienced in shoulder reconstructive procedures
should always be considered for more complex injuries. This should
particularly apply if local resources are limited or if the attending
surgeon considers that they lack the experience to adequately treat the
injury. Ideally, these injuries should be treated by surgeons who are
experienced in both modern fracture reconstructive techniques and
arthroplasty.
of the existing literature have highlighted the paucity of Level I, II,
or III evidence. The quality of the published literature on the
treatment of proximal humeral fractures is heterogeneous, and most
studies are retrospective Level IV and Level V case-series studies. The
patient group being described is often ambiguous, because of
inconsistencies in classification and patient selection. Most series
are also too small to support any statistical conclusions, and patients
with different injuries are often combined to increase group size.
General applicability may be uncertain as reports are often from a
single surgeon reporting a single technique. Furthermore, the bulk of
the published results emanate from centers of excellence, where the
patterns of injury encountered may be different from everyday practice.
The excellent results reported by shoulder specialists, working with
modern facilities in tertiary referral centers, may or may not be
reproducible in centers where expertise and resources are more limited.
Inevitably, most papers report
successful
results and this may not reflect the generality of experience with a
particular treatment. Many of the operative series have reported on
younger patients with more innocuous injuries, as a result of the
selection bias and the same results may not be achieved in the elderly.
Very few studies directly compare different treatment modalities, and
there are only three randomized trials of surgical intervention in the
literature.164,289,324
 |
|
FIGURE 35-21
The extended deltoid-splitting approach uses a distally based skin flap to improve cosmesis and allow extension into the deltopectoral interval if required (A). Formation of the elliptical skin flap allows full exposure of the superior deltoid muscle, which is then split, creating an “upper window” proximal to the axillary nerve. A large artery forceps is inserted through the deltoid distal to the area of the nerve and is used to deliver an arterial sling around a cuff of soft tissue containing the axillary nerve (B). The “lower window” of the incision is created distal to the arterial sling protecting the axillary nerve by continuing the deltoid split, which then allows visualization of the lateral proximal humeral diaphysis with further dissection. The lower window is then exposed using hand-held retractors to allow insertion of lower plate screws into the proximal humeral diaphysis (C). (continues) |
assessment of pain, range of movement, and ability to perform normal
activities, which are usually collectively amalgamated to produce a
functional score. This score is then used to grade the outcome, usually
into the four categories of “excellent,” “good,” “fair,” and “poor.”
Some scores also incorporate a radiological assessment, but most
studies consider this separately. The other surrogate outcome measure
which has been used is the incidence of complications.
ASES scores) is that they are not sufficiently flexible to accommodate
the variability in patients’ own expectations from treatment. Entirely
different results for the same patients may be obtained depending on
the scoring system used.34
Function is closely related to age and activity, and an outcome
resulting in disability in a young and active patient may equate with
entirely satisfactory function in the elderly patient.181,196,316
More recently, newer audit tools have been produced, which attempt to
address this deficiency by assessing the patient’s own aspirations and
feelings about their injury and its treatment. The newer tools are
administered as questionnaires, which either selectively assess limb
function (the DASH score and the Oxford score) or assess the patient’s
general health status in response to injury (the SF-36 and
Musculoskeletal Function Assessment). Patients’ assessments of their
own outcomes are often significantly more favorable than their
numerical scores would suggest.125,236,323
The chief drawback of these is the difficulty in assessing the
patient’s functional status before their injury, to evaluate the
efficacy of the treatment used. Although normative “control” values are
available for the general population, the patients who sustain proximal
humeral fractures may not be strictly comparable.
 |
|
FIGURE 35-21 (continued)
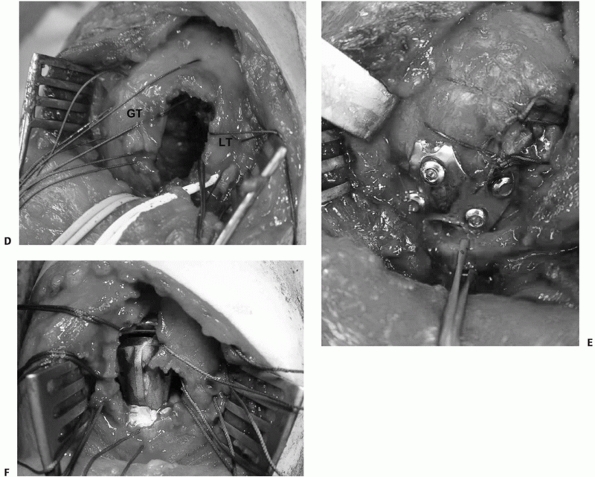
The tuberosities (GT and LT) are tagged and the fracture is reduced, creating a large cancellous void between the tuberosities, which requires bone grafting (D). Fractures may be treated by either internal fixation (E) or hemiarthroplasty (F) using this approach. |
|
TABLE 35-3 Summary of the Medical Comorbidities Commonly Associated with Increased Risk of Surgical Complications
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
reproducibly assess components of shoulder function, but these have not
been widely used in clinical studies to date.144
Assessment of muscle strength and range of movement can also be made
using commercially available muscle testing machines, which can
selectively “isolate” muscle groups and produce computer-generated
simulations of normal daily activities. The range of movement,
strength, and endurance of the injured shoulder are compared with the
normal uninjured side, thereby “controlling” for the effect of age and
individual variation. These systems have the advantage of providing a
quantitative evaluation
of
function, and repeated testing allows appraisal of the recovery of
function over time. The major drawback is that objective evidence of
weakness may not correlate well with the patient’s overall level of
function and degree of satisfaction with their outcome. In addition,
isolation of the individual components of shoulder movement is
difficult because of the confounding affects of coexistent
scapulothoracic movement. Nevertheless, this technology may prove to be
increasingly useful in the future in the objective assessment of
functional outcome.
|
TABLE 35-4 The Advantages and Disadvantages of the Techiques Used to Treat Displaced Proximal Humeral Fractures
|
|||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
union, the degree of malunion, and the presence of osteonecrosis and
degenerative change. Assessing union may be difficult in many fractures
because of the metaphyseal location of the fracture, which seldom
results in much external callus formation. Union is therefore usually
implied by lack of displacement of the fracture on sequential
radiographs and diminution rather than widening of fracture lines. This
is supported by lessening of shoulder pain and return of function.
Assessment of union after anatomic reduction and internal fixation is
even more difficult, although fixation failure or progressive loosening
is always associated with nonunion. CT is useful in doubtful cases.
Malunion may be assessed by measuring residual displacement of fracture
fragments with respect to each other using conventional radiographs,
but accurate assessment can only be made using CT.
partial head involvement to complete involvement and collapse. There is
currently no generally accepted classification for osteonecrosis in the
humeral head. The differential diagnosis is from osteoarthrosis of the
head, which is classified using the system of Samilson and Prieto.267
Collapse and deformity are usually not present in osteoarthrosis. CT
and MRI are useful where there is doubt about the diagnosis and to
assess the degree and severity of head involvement.
during both operative and nonoperative treatment. The three major
complications of treatment are nonunion, osteonecrosis, and rotator
cuff dysfunction and stiffness. After operative treatment, one quarter
of patients can expect to develop one or more significant complication
during treatment, with 1 in 10 requiring further surgery. Complications
of treatment may be inaccurately reported in retrospective studies,
because of incomplete documentation in case records. Prospective cohort
studies usually report higher incidences, and this may explain the wide
variation in the published literature.
treatment, and these are discussed at the end of the chapter.
Complications that are specific to a particular method of treatment are
discussed in the following treatment section. There are identifiable
factors associated with an increased risk of specific complications (Table 35-5).
than particular fracture types. For this reason, in the following
section treatment is discussed by technique rather than by fracture
configuration.
have undisplaced or stable osteoporotic proximal humeral fractures,
will have a pain-free shoulder that is functional to their requirements
after nonoperative treatment, if they avoid the major complications of
nonunion, osteonecrosis, and rotator cuff dysfunction. There is
evidence to suggest that continued functional recovery may take place
during the first 2 years after the injury.
However,
the rate of recovery is rapid in the first 6 months, slows
exponentially during the subsequent 6 months, and is minimal in the
second year.244,253,257
Complete functional normality is uncommon, but most patients have only
minor complaints of activity-related ache and sensitivity to cold or
damp weather (“barometric shoulder”).
|
TABLE
35-5 Factors Associated with a Poorer Functional Outcome and Increased Risk of Complications after a Proximal Humeral Fracture |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
of patients with more complex fracture configurations, and those who
are younger have higher functional demands on their shoulder. Careful
preoperative counseling is therefore required, because the functional
benefits from surgery are usually relative rather than absolute, and
there is a substantial risk of complications.
of feeding, washing, and maintaining perineal hygiene requires only a
composite (at the glenohumeral joint and scapulothoracic articulation)
pain-free arc of 60 degrees forward flexion, abduction, and internal
rotation at the shoulder girdle. Some authors have suggested that such
a result may be considered satisfactory in the elderly.196,316
Even lesser degrees of movement may be tolerated in low-demand elderly
individuals, whereas more movement is often required for function in
younger, active patients. A wide range of intrinsic and extrinsic
factors have been associated with poorer functional outcomes from
treatment (Table 35-5).
approximately 85% of proximal humeral fractures. The absolute
indications for its use include minimal displacement (one-part
fractures), advanced age (usually over the age of 85 years), dementia,
and medical contraindications to operative intervention. However, in
the majority of instances, nonoperative treatment is selected because
in the surgeon’s opinion the fracture pattern is stable. This usually
implies that there is residual cortical contact between the shaft and
the humeral head fragment, preferably with some impaction of the two
fragments, and minimal tuberosity displacement. Such a configuration
substantially reduces the risk of later nonunion, regardless of whether
the fracture is of two-, three-, or four-part configuration.
after the injury, because adequate splintage across the fracture is not
possible. The pain is typically aggravated by recumbency, and most
patients find it most comfortable to sleep in a chair or recliner for
the first week after the injury. In elderly patients, admission to
hospital may be required, because many will be unable to cope with
their normal daily activities, especially if they are living alone.
therapy, is prescribed, and a sling is used to rest the arm, which
allows distraction of the fractured bone ends.66 Hanging casts offer no demonstrable advantage181,236,290
and may excessively distract the fracture, predisposing to nonunion.
Prolonged immobilization does not improve healing rates or outcome,156 and the functional recovery is better when physiotherapy commences early.47,156,164,181,316
elbow, wrist and hand mobilization should begin immediately, with
assisted passive shoulder rotation, flexion, and abduction (“pendular”)
exercises beginning at around 1 week.127
Active shoulder isometric exercises are begun at 3 weeks, progressing
to isotonic strengthening and stretching exercises at 6 to 12 weeks.79,156
It is important that exercises continue during the first year after
injury, because delayed restoration of movement may continue during
this time. Prolonged supervised physiotherapy is often recommended,156,181
although there is evidence that a single physiotherapy session with
subsequent performance of exercises at home is just as effective.20 Physiotherapy adjuncts, such as hydrotherapy242 or pulsed electrotherapy,188 do not improve outcome.
fractures, which are at risk of secondary redisplacement by repeat
radiographs in the first 2 weeks. It is not uncommon to see transient
inferior subluxation of the humeral head in stable fractures, which may
be caused by hemarthrosis, deltoid inhibition, or rotator cuff
dysfunction233,315 (Fig. 35-22).
Late radiographic monitoring should ideally be undertaken to ensure
fracture healing and absence of signs of osteonecrosis during the first
few years after the injury. In practice, this is seldom practicable and
most patients are discharged from follow-up once their fracture is
healed and they have regained satisfactory function.
of patients with one-part fractures involving the surgical neck of the
humerus,47,86,144,156,164,196,316 one-part greater tuberosity fractures,229 impacted two-part surgical neck,58 varus two-part surgical neck,59 and three- and four-part impacted valgus56 fractures treated nonoperatively (Table 35-6).
Union rates of up to 100% have been reported for minimally displaced
fractures, with the vast majority of patients achieving a painless or
only mildly painful shoulder.56,58,59,86,156 The final range of motion achieved is up to 90% of the uninjured side,156 and most patients are satisfied with their outcome.316 The radiographic appearance often fails to correlate with the final clinical outcome.197,316
 |
|
FIGURE 35-22 Inferior subluxation of the humeral ahead is not uncommon after impacted surgical neck of humerus fracture (A). This usually corrects spontaneously during the first 2 weeks after the injury (B).
|
However, several of these studies comment on the high degree of patient
satisfaction despite the poor functional score and poor radiologic
appearance after treatment.236,323
The studies that have compared nonoperative with operative treatment
have produced conflicting results. Some show a benefit from operative
intervention,161 whereas the majority fail to demonstrate convincing benefits from operative intervention.58,78,324
fractures, nonunion, osteonecrosis, and symptomatic malunion, are the
major complications which may occur after nonoperative treatment (Table 35-6). The risk of these complications is extremely low when nonoperative treatment is used for minimally displaced fractures316 but is higher for older patients and those with displaced and multipart fractures.181,182,220,273
Failed nonoperative treatment is not benign and subsequent operative
reconstructive procedures are usually technically more difficult,
resulting in a poorer outcome overall than when the operative
intervention is performed primarily.34
treatment, surgery can be deferred for 48 hours without deleterious
effects. If the patient has medical comorbidities, it is best to wait
for these to be optimized in the first week after injury before the
surgery is performed. There is no evidence to suggest that the timing
of surgery affects the outcome. All procedures are performed under
general anesthesia with broad-spectrum antibiotic and antithrombotic
prophylaxis. A scalene block is often performed for postoperative
analgesia.
|
TABLE
35-6 Summary of the Case Series Results of Nonoperative Treatment for Proximal Humeral Fractures in Series That Report Functional Outcome* |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
fracture surgery. The patient is positioned in a supine beach-chair
position, with a head rest and shoulder operating table “cut-away” to
facilitate access for the fluoroscope from the opposite side of the
table (Fig. 35-23). By rotating the C-arm, good anteroposterior and “modified” axial views302 can be obtained during surgery.253,254
It is important to secure all anesthetic tubing and make sure that
equipment is cleared from the path of the C-arm to prevent
intraoperative detachment. The whole arm is prepared and draped, to
allow its free movement by the assistant during surgery. Appropriate
instrumentation for both humeral head fixation and replacement should
always be available in the operating theatre (Table 35-7).
The potential advantages of these techniques are the same as for any
other minimally invasive surgery, by improving cosmesis and reducing
blood loss, postoperative pain, infection risk, and trauma to adjacent
soft tissues. In the shoulder, avoiding surgical injury to the deltoid
muscle is seen as a particular advantage because of the risk of
dysfunction and pseudoparalysis of this muscle that can develop after
open surgery. However, most of these techniques are technically
demanding and have a protracted learning curve.
treatment under general anesthesia has been used to try to reduce
displaced fractures. Typically an attempt is made to impact the shaft
fragment onto the humeral head. Reduction of displaced tuberosity
fragments cannot be achieved. Unfortunately, this technique is
associated with a very high rate of redisplacement of the fracture
fragments, even after a good initial reduction.196,208,289,316
The technique is now seldom used alone, without some attempt at
improving the stability of the reduction, typically using percutaneous
fixation.
 |
|
FIGURE 35-23
The patient is positioned in the beach chair position for surgery. The fluoroscope is positioned on the opposite side of the operating table (A), to allow the surgeon and assistant to have free access to the shoulder throughout the procedure (B). |
techniques have been described to treat most types of displaced
proximal humeral fractures, with the exception of
fracture-dislocations. Valgus three- and four-part fractures, and
unimpacted two-, three-, and four-part fractures in which the shaft has
translated or separated from the head fragment, are thought to be
particularly suitable for the use of this technique.*
reduction is achieved under C-arm fluoroscopy, using a combination of
closed manipulation and percutaneous introduction of instruments and
pins to act as “joysticks” that manipulate fracture fragments. Detailed
knowledge of the local anatomy is required to facilitate reduction and
to avoid damaging the anterior branch of the axillary nerve, which is
vulnerable to injury as it courses around the neck of the humerus
laterally. Anatomic studies have confirmed the close relationship of
the axillary nerve, long head of biceps, circumflex humeral vessels,
and cephalic vein to the pins.138,262
achieved using threaded K-wires or cannulated screws inserted across
the key fracture fragments122,133,240 (Fig. 35-24).
Smooth and thin wires have a tendency to loosen, break, or migrate, and
thick, terminally threaded K-wires are used to minimize the risk of pin
migration.122,152
The pins are inserted through small incisions, and a curved hemostat is
used to dissect down to the periosteum and to mobilize soft tissue to
avoid neurovascular injury. A protective sheath such as a drill guide
should be used to prevent injury to the soft tissues. Two or three
retrograde pins are placed from the shaft into the head fragment. The
pin
entry
point should be distal enough to the fracture line to gain adequate
purchase of the intact humeral shaft cortex. The pins are inserted
eccentrically to each other, from anterolateral to posteromedial (about
30 degrees), because of the anatomic retroversion of the humeral head.
|
TABLE 35-7 Surgical Pearls: “Checklist” for the Reconstruction of Proximal Humeral Fractures
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
wires or cannulated screws. Pins are placed under fluoroscopic guidance
through the greater tuberosity, from superolateral to inferomedial,
approximately 1 cm distal to the rotator cuff insertion, engaging the
medial cortex of the shaft fragment. At this point, care should be
taken not to advance the pins past the medial cortex to avoid damaging
the axillary nerve or posterior humeral circumflex artery. The pins are
cut below the skin to reduce the risk of pin tract infection.
individual wires and screws is often poor, and percutaneous fixation
does not provide absolute stability. To enhance the stability of the
reconstruction, a “humerus block” may be inserted in a minimally
invasive manner, which links together the head-shaft wires, improving
the mechanical stability of the reconstruction.238
It is noteworthy that the use of this technique tends to be reported in
selected younger patients, who would be expected to have a lower rate
of these complications from operative treatment.
repair rotator cuff tears have been adapted for the acute reduction and
internal fixation of isolated lesser and greater tuberosity fractures22,40,99,141,148,271 and selected more complex fractures.6,63,99,141,143,297
This technique is promising, allowing minimally invasive direct
visualization of the reduction of the displaced fragments rather than
the indirect view afforded with percutaneous fixation under C-arm
fluoroscopy. However, there is a protracted learning curve, and
expertise in arthroscopic surgery is required.*
Most of the published literature in this area is in the form of
isolated case reports at present, and it is uncertain whether there are
discrete advantages of their use over open surgical techniques.
 |
|
FIGURE 35-24
Percutaneous pin fixation may be used to treat a variety of displaced two-, three-, and four-part fractures. In an impacted-valgus fracture, the humeral head is reduced with a percutaneously introduced bone tamp (A). The greater tuberosity is pulled inferiorly with a clamp or a hook (B). The fracture reduction is maintained with percutaneous pins in a standard configuration (C,D). Alternatively cannulated screws may replace the wires if the bone quality is better (E,F). (C, From Jaberg H, Warner JJP, Jakob RP. Percutaneous stabilization of unstable fractures of the humerus. J Bone Joint Surg Am 1992;74A:508-515, with permission.) |
|
TABLE
35-8 Summary of the Case Series Results of Minimally Invasive Techniques for Proximal Humeral Fractures in Series That Report Functional Outcome* |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Many of the modern locked-plating and intramedullary nailing systems
that have been introduced in the past 10 years have the facility for
percutaneous insertion through small stab incisions. Screw insertion
proximal and distal to the fracture is performed percutaneously using
custom-made jigs. The techniques of reduction are the same as those
used for percutaneous pin/screw fixation, and similar local soft tissue
structures are at risk of injury. This technique tends to be reserved
for simpler displaced two-part surgical neck fractures, where reduction
of the two main fragments can be accomplished relatively easily.170,284
However, the technique is now seldom used because of the poor fixation
gained by half-pins in the humeral head, the high risk of pin track
infection, and the availability of more rigid methods of definitive
fixation. The Ilizarov method had also been used as a primary treatment
for proximal humeral fractures132,275 but is now usually reserved for the treatment of contaminated open fractures140 and occasionally for nonunions with large bone defects.42
invasive approaches, because of the shorter learning curve, greater
accuracy of articular reduction, and greater stability of fixation that
can be achieved using more rigid internal fixation implants. The major
drawbacks include the greater blood loss and risk of infection after an
open surgical exposure. A large variety of implants have been used to
provide definitive fixation, and techniques that have been used in more
than one case series are discussed further.
is currently the most commonly used method of internal fixation for
displaced proximal humeral fractures. The aim is to provide secure
fixation of the humeral head to the shaft, thereby preventing secondary
torsional redisplacement of the shaft from the head and to provide
additional fixation of reduced tuberosity fragments. This technique is
the most widely used method to treat displaced but salvageable two-,
three-, and four-part fractures. Several key steps are identified,
which are critical to the success of the reconstruction.
The procedure can be performed through a deltopectoral or extended
deltoid-splitting approach, identifying and protecting the anterior
branch of the axillary nerve before any fracture manipulation or
instrumentation. In three-part greater tuberosity and four-part
fractures, the main split in the tuberosities is first located in the
area immediately posterior to the bicipital groove. The space formed by
this fracture line is developed to gain access to the humeral head and
the glenohumeral joint. Both tuberosities are tagged with nonabsorbable
interosseous sutures. If the bone quality is poor, these sutures should
be placed intratendinously, through the rotator cuff tendon insertion
in the tuberosity. It is customary to extend the split in the
tuberosities by incising along the line of the rotator interval, to
perform a limited arthrotomy. This allows inspection of the articular
surface to assess any marginal articular fragments attached to the
tuberosities, and any other articular pathology. In displaced two-part
surgical neck fractures, none of these steps are required, unless there
are undisplaced tuberosity fracture lines, which should be sutured at
this stage to prevent later redisplacement.
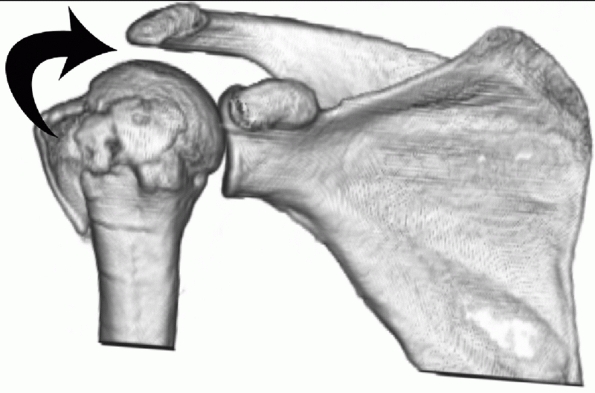 |
|
FIGURE 35-25 In an impacted-valgus fracture, the humeral head has to be elevated to achieve reduction (arrow).
|
Regardless of the fracture configuration, reduction of the humeral head
onto the shaft fragment must be achieved in all three planes of
potential displacement (sagittal, coronal, and horizontal).
Preoperative three-dimensional CT greatly facilitates understanding of
the pattern of displacement and planning the reduction maneuvers. It is
customary to reduce the fracture in the sagittal and coronal planes
first: Varus and valgus deformities of the head can be corrected by the
eccentric insertion of K-wires, elevators, or osteotomes into the
humeral head (Figs. 35-25 and 35-26).
These are then used as joysticks to correct the deformity. In the
sagittal plane, it is customary for the head to tilt into slight
flexion or extension, which is usually fairly easily corrected.
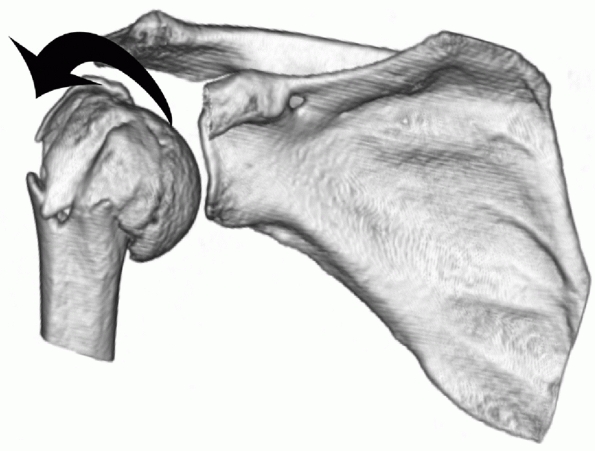 |
|
FIGURE 35-26 In a varus fracture, the humeral head has to be elevated from its inferiorly subluxed position to achieve reduction.
|
anatomic reduction can usually be achieved by correcting any
translation of the shaft under the head. The shaft is
characteristically displaced anteromedially, because of the pull of
pectoralis major. Reduction is usually achieved by lateralizing the
shaft, either directly by applying reduction forceps or indirectly by
pressure in the axilla, using a custom-made “bolster,” which helps
avoids excessive manual handling in this potentially unsterile area.254
Alternatively, there are a variety of indirect reduction techniques
using the definitive plate osteosynthesis to “fine-tune” the reduction287 (Fig. 35-27).
undertaken. In two-part fractures, correct interdigitation of fracture
lines in the head relative to the shaft confirms correction of any
rotational deformity. In three- and four-part fractures, correction of
rotation can be confirmed by ensuring satisfactory alignment of the
bicipital groove (containing the biceps tendon) across the fracture.
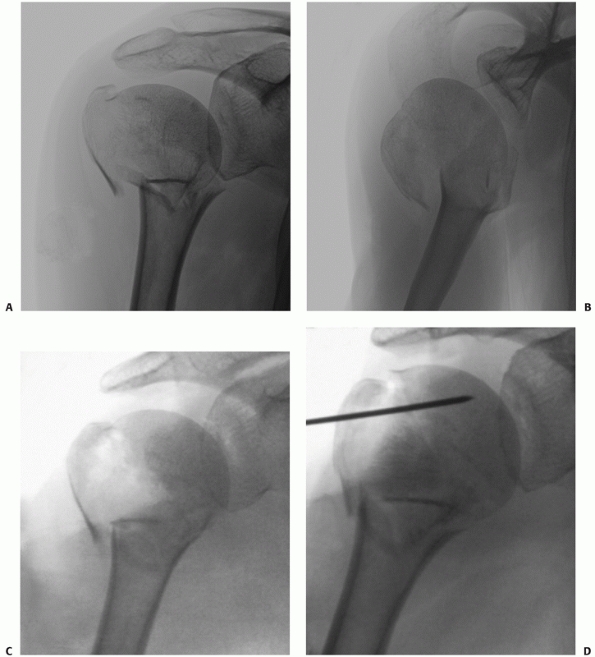 |
|
FIGURE 35-27
Indirect reduction techniques may be used to reduce fractures in which there is appreciable medial translation of the shaft fragment from the head (A,B). The head is disimpacted and reduced, leaving a large metaphyseal cancallous void (C), which is filled with femoral head allograft (D). (continues) |
humeral head must be relocated into the glenoid before these reduction
maneuvers can be performed. In Type I anterior-fracture dislocations
and posterior fracture-dislocations, the humeral head must be first
disengaged from the anterior or posterior glenoid rim, respectively,
under direct vision. Relocation can then usually easily be achieved by
rotation of the humeral head into the glenoid. In Type II anterior
fracture-dislocations, the humeral head is not engaged on the glenoid.
Relocation of the humeral head is achieved by delivering it back
through the tear in the
anteroinferior
capsule. Occasionally, the mobile humeral ahead cannot be relocated
through the extended deltoid-splitting approach and a supplementary
deltopectoral approach is required.
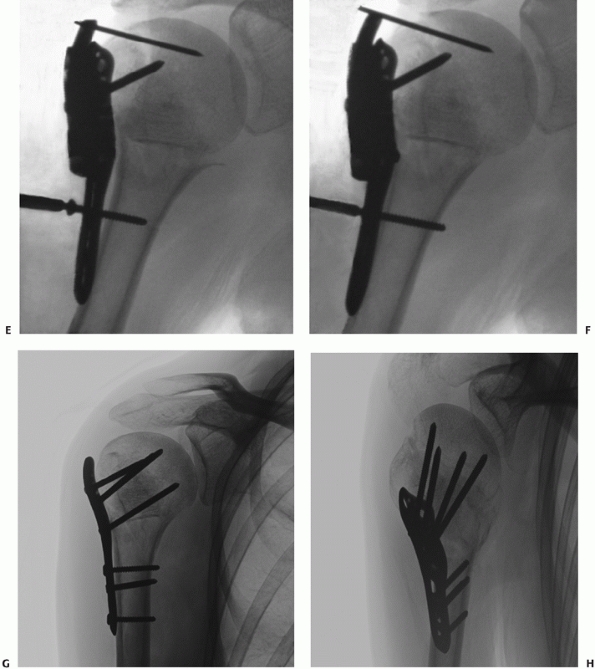 |
|
FIGURE 35-27 (continued) A locking plate is applied and secured to the head fragment with no attempt to reduce the shaft (E). Tightening a nonlocking screw distal to the fracture translates the shaft laterally to achieve reduction (F). The remaining head and shaft screws are now inserted to produce the final reconstruction (G,H).
|
Once the head is reduced, it can be temporarily held with K-wires. In
three- and four-part fractures, there is often an extensive metaphyseal
void after reduction of the displaced humeral head fragment, which is
then irrigated clear of blood and debris. The cancellous void may be
ignored, but there is a trend toward filling these defects with bone
graft or substitutes, to enhance stability and reduce the risk of
redisplacement. Small cancellous defects (less than 20-mL) have been
filled using bone substitutes, which are injected in a semiliquid form
and cure over a period of 10 to 20 minutes (Fig. 35-28).
Where there is a greater metaphyseal void, impacted morselized
autograft or allograft and structural allografts have been used to fill
the defects106,253,254,263,296 (Fig. 35-29).
the stability of reduction has also recently been highlighted. If this
area is fractured and comminuted, or is malreduced, there is potential
for instability and acute redisplacement despite internal fixation.
This frequently applies to two-, three-, and four-part fractures, where
the head has displaced into varus. This is analogous to the
posteroinferior comminution seen in intracapsular femoral neck
fractures, which is frequently implicated in
acute
fixation failure after reduction and screw fixation. Where this support
is compromised in proximal humeral fractures, the use of either a low
inferomedial screw (Fig. 35-29) or a fibular strut graft has been advocated to restore stability.91,95,97
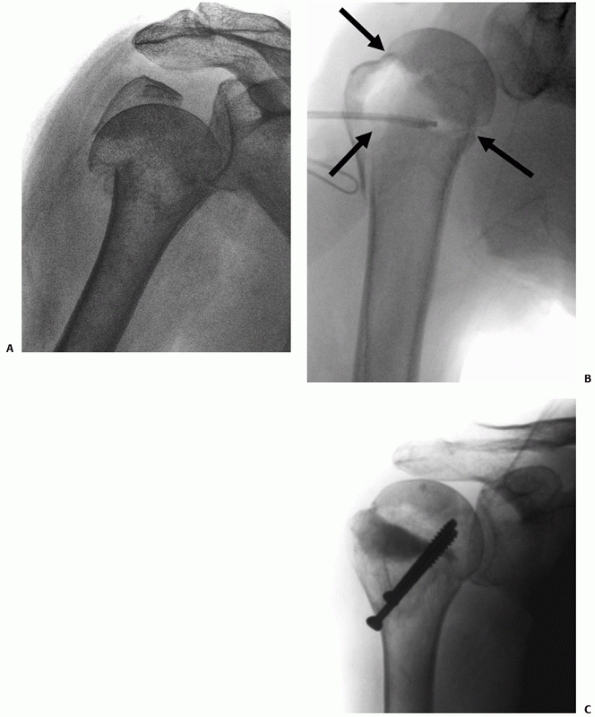 |
|
FIGURE 35-28 Large laterally based triangular metaphyseal defects are created after reduction of impacted valgus fractures (A,B). These can be filled with bone substitutes if the defect is small (C) or bone graft if the defect is more than 20 mL.
|
reverse Hill-Sachs lesion associated with a two-, three-, or four-part
posterior fracture-dislocation, which may lead to acute redislocation
as the defect reengages on the posterior glenoid rim. Where there is a
large residual osteochondal fragment with retained soft tissue
attachment, this is treated by elevation of the osteochondral fragment,
with insertion of morselized femoral head allograft to buttress the
elevated fragment, with support from “positional screws.”244,245 Where the fragments are comminuted or loose, a femoral head osteochondral allograft is sculpted to fill the defect.102,244,245
Bone grafting of classic Hill-Sachs lesions associated with anterior
fracture-dislocations is not usually required, because the shoulder is
usually stable once reduced.
metaphyseal defects is to span the defect by inserting a modular
humeral arthroplasty stem. The humeral head is then reattached to the
cemented stem using a “Bilboquet” device, which is a circular staple,
which is driven into the raw cancellous surface of the back of the
humeral head.70
reduced and fixed. It is important to check at this stage that any
marginal articular surface fragments attached to the tuberosities are
reduced anatomically (Fig. 35-30).
If these are unstable, supplementary subchondral screw or threaded wire
fixation may be required to maintain stability. It is also important to
confirm that both tuberosities are anatomically reduced relative to the
humeral head and shaft.
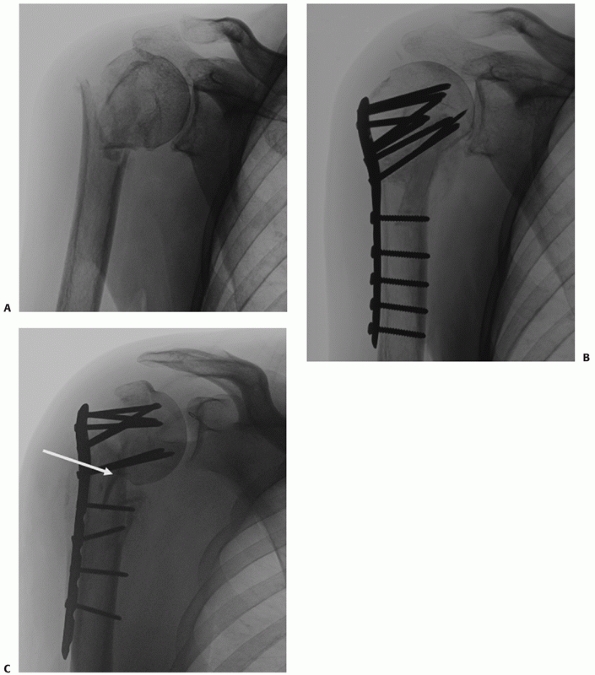 |
|
FIGURE 35-29 The defect produced after reduction of a varus displaced fracture (A)
is larger on the side of the medial calcar (medially based triangular defect). These fractures require support from structural bone graft and low calcar screws as show in this case (B). In another case, failure to provide adequate calcar support (arrow) resulted in early failure of the locking plate with the head falling back into varus (C). |
early “pull-off,” using cancellous screws or an interosseous suturing
technique, dependent on the bone quality and the extent of comminution(Fig. 35-31).
Securing a tuberosity in a superior position may give rise to rotator
cuff impingement, and advancing it too distally may defunction the
rotator cuff.
It is important to appreciate that the function of the plate is only to
reconnect the head to the shaft. Tuberosity fragments must be fixed
separately, because the fixation of these fragments provided by screws
inserted through the plate will not provide sufficient stability to
prevent their redisplacement. Generic cloverleaf, buttress, and simple
semitubular plate fixation have been successfully used to treat both
simple and complex proximal humeral fracture patterns for many years (Table 35-9).
Over the past 10 years, proximal humeral locking plates have been
introduced, which may help to provide more secure fixation in the
osteoporotic bone of the humeral head, theoretically reducing the risk
of fixation failure.23,76,154,202
Many different designs of plate are now available, differing in their
shape and the locking mechanism at the screw-plate interface.303,304
Some plates permit only a fixed (monoaxial) orientation of each screw
to the plate using a jig, while others allow a degree of freedom
(polyaxial) in the angle at which the screws can be inserted.
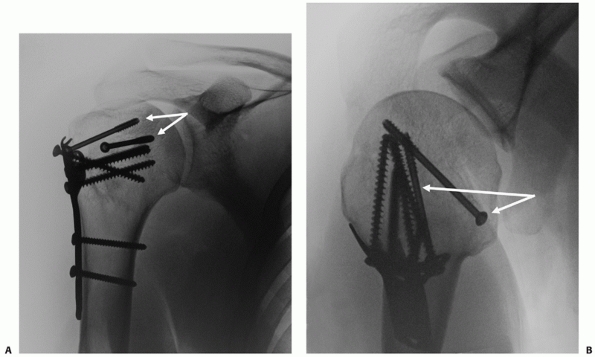 |
|
FIGURE 35-30 A,B. Articular surface fractures must be reduced and fixed separately using screws (arrow).
|
and the orientation of the plate relative to the reduced greater
tuberosity is critical: The anterior free edge of the plate should be
placed just posterior to the bicipital groove to facilitate the
insertion of the screws centrally or slightly posteriorly in the
humeral head, which is offset posteriorly and retroverted relative to
the shaft. The plate must be cranial enough to allow screws to be
inserted centrally into the head in the anterior projection but not so
high that the top edge of the plate impinges against the acromion. The
plate may be used to indirectly “fine-tune” the fracture reduction (Fig. 35-27).
 |
|
FIGURE 35-31
The tuberosities should be reduced and fixed with sutures, as well as screws through the plate to prevent tuberosity pull-off as depicted previously in Figure 36-21E. Failure to securely fix the greater tuberosity usually results in early redisplacement, as shown (arrow). This usually produces a symptomatic rotator cuff-deficient shoulder. |
the fracture, with four to six head screws proximally, dependent on the
bone quality. It is essential to ensure that none of the head screws
are inadvertently placed within the joint. The drill is inserted at low
speed using a “push” technique until it can be felt to encounter
resistance when it comes into contact with the hard subchondral bone of
the humeral head. The screw length is measured at this point and should
be between 40 and 50 mm in most cases. Once all screws are inserted,
the shoulder is rotated under live fluoroscopy in both anteroposterior
and modified axial views, to ensure no intra-articular screws are seen.
series focus on technical aspects of plate treatment and contain mixed
subgroups of fractures. This prevents detailed analysis of the results
for individual fracture subtypes using these implants (Table 35-9).
The functional results are satisfactory using both conventional and
locking plates, with a relatively low rate of the major complications
of osteonecrosis, nonunion, and malunions. As a result, the rate of
revision surgery is comparatively low in most series.
It is apparent that stable reduction of the fracture is still required
to prevent the risk of later fixation failure of these stiffer implants
at the implant-bone interface.183
Revision of the operative technique, with the increased use of bone
grafts or supplementary calcar screws to improve the stability of the
reduction, may result in further improvement in results.
|
TABLE
35-9 Summary of the Case-Series Results of Plate Fixation Techniques for Proximal Humeral Fractures in Series That Report Functional Outcome* |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
nailing of proximal humeral fractures has the advantage of reducing the
surgical exposure required to obtain secure fixation. However, despite
the more lateral entry point of some nails, there are concerns about
the injury to the rotator cuff that is inevitably produced during nail
insertion,186 as for nailing of humeral diaphyseal fractures.246
Due to the reduced fixation options proximal to the fracture, nailing
has been mainly used to treat displaced two-part surgical neck
fractures.18,186,235
Newer nail designs allow multiple proximal screws to be inserted
eccentrically through the nail, to fix three- and four-part fractures.
Locking technology has also been used to allow screws to lock into the
nail, improving the rigidity of the reconstruction.62,185,198
However, when the greater tuberosity is fractured, there is a greater
risk of redisplacing the fracture during nail insertion, and the
results in more complex fractures have been poorer with a 45%
reoperation rate.3,18
For this reason, the current “ideal” indication for this technique is a
more elderly patient with a displaced two-part surgical neck fracture,
and the implant is not often used to treat more complex three- and
four-part fracture configurations.
where the nail is used to treat a fresh, displaced surgical neck
fracture, a smaller deltoid-splitting incision is used to gain access
to the nail entry portal. After a miniarthrotomy has been made through
the supraspinatus, dependent on the nail design, the entry point for
the nail is either through the anterior portion of the greater
tuberosity or through the lateral articular surface just adjacent to
it. After opening into the medullary canal, a guide wire is screened
across the reduced fracture. Minimal reaming is usually required and
the nail is the inserted by gentle pushing and twisting movements.
Using a radiolucent targeting jig, proximal and distal locking screws
are inserted (Fig. 35-32). The arthrotomy and rotator cuff are meticulously repaired at the end of the procedure.
infrequently for displaced extra-articular surgical neck fractures.
They have the advantage of distal insertion, thereby avoiding
dissection of the rotator cuff and capsule. Results have been reported
from a number of centers internationally using Ender nails,219 large K-wires,234 and specially designed flexible pins318,319,320 and nails110 in the treatment of two-part neck fractures. Unfortunately, functional results are available from one only study.234 The main complication of wire insertion (via the olecranon fossa) is restriction of elbow extension,219 but early implant failure necessitating revision surgery is also reported in 25% of patients.319
However, most series report substantial rates of fixation failure and
malunion, especially for more unstable two-part fractures and multipart
fractures.3 There is also a
substantial risk of rotator cuff dysfunction requiring secondary nail
removal. These complications result in a high rate of revision surgery
after the use of these implants.3
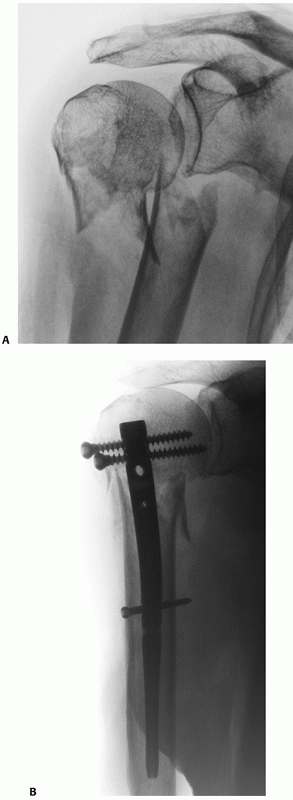 |
|
FIGURE 35-32 Translated and comminuted displaced surgical neck fractures (A) can be treated by locked intramedullary nailing in the older patient (B).
|
principles are theoretically highly applicable in the proximal humerus
where the tensile force of the rotator cuff attachments to the
convexity of the anterolateral humerus can be converted into
compressive forces. Screw fixation either alone or combined with
interosseous suture fixation techniques may be used in isolated
two-part tuberosity fractures and some minimally displaced three- and
four-part fractures.106,253,254,296
|
TABLE
35-10 Summary of the Case Series Results of Intramedullary Nailing Techniques for Proximal Humeral Fractures in Series That Report Functional Outcome* |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
However, wire sutures are prone to breakage and loosening, and the
technique was subsequently adapted with the incorporation of other
implants such as Enders nails, K-wires, and supplementary screws to
improve the rigidity of the reconstruction in osteoporotic bone.247,306
The latest generation of high-strength cords and braided suture
materials have now replaced wiring in the modern-day version of this
technique.67,106,217,227
Other adaptations have also been described to improve the mechanical
stability of the reconstruction in osteoporotic bone, including
impaction of the humeral head, a “parachute” suturing technique,9 and augmentation of the fixation with external fixation.151
Regardless of the technique used, it is important to stabilize the head
to the shaft and fix any tuberosity fractures to both the head and the
shaft.
These techniques have been mainly used in the elderly, where
conventional plate fixation was thought to be at high risk of failure
from screw cut-out. It remains to be seen whether these procedures will
continue to have a role, with the recent advances in plate and
biomaterial technology to provide more stable fixation in osteoporotic
bone.
osteonecrosis and nonunion was reported to be prohibitively high after
three- and four-part fractures, with or without dislocation of the
humeral head.207,209
Neer popularized the use of replacement arthroplasty to treat acute
fractures in the early 1970s, and this remains the main indication for
the use of this technique today. Delayed arthroplasty has also been
widely used to treat complications arising from both nonoperative and
operative fracture treatment.5,28
total shoulder arthroplasty, except where the patient has degenerative
joint disease affecting the glenoid. It is also customary to use
cemented rather than uncemented implants, because of the poor potential
for bone-implant integration in these osteoporotic fractures. The
initial stemmed implants, such as the Neer prostheses (Mark I and II),
were of monoblock design, but these have been superceded by modular
designs, which allow adjustment of the height and offset of the
prosthesis after cementation of the stem. Newer implants, such as the
Tournier prosthesis, are now available that are specifically designed
to treat fractures and have jigs for judging insertion, porous coating,
and fenestrations to encourage tuberosity healing.
relationship of the humeral head to the tuberosities and to the shaft
by achieving proper component height, off-set, and version. Anatomic
healing of the tuberosities is often associated with a good functional
outcome, while secondary displacement or nonunion is usually associated
with a weak and poorly functional shoulder. It is therefore critical
not only to ensure optimal fixation of the tuberosities but also to
prevent secondary displacement by postoperative protection of the
shoulder. Postoperative stiffness in the presence of tuberosity healing
is a manageable problem, whereas secondary tuberosity displacement and
nonunion are untreatable.
The patient is positioned in the beach-chair position, preferably with
a cut-away to facilitate a full intraoperative range of movement of the
shoulder. All procedures are performed under a standard antibiotic and
antithrombotic prophylaxis regimen. The initial surgical approach is
identical to that for an internal fixation procedure and can be
achieved using either a deltopectoral approach or a deltoid-splitting
approach. The tuberosities are mobilized, and heavy nonabsorbable
braided sutures (Ethibond, Fibrewire, or Orthocord) are placed through
the tendon around each tuberosity. The humeral head is extracted and is
used to select the head implant size.
preoperative templating, or the calcar of the proximal humerus, which
serves as a guide to the location of the anatomic neck of the humerus
before the fracture. If the calcar is fractured, another useful
internal reference point is the upper border of the pectoralis major
insertion. The average distance from this point to the top of the
humeral head has been shown to be 5 cm.100
The greater tuberosity can also be used to check the height of the
implant, as the neck of the prosthesis will have to lie exactly at the
level of the insertion of the supraspinatus if it is anatomically
reduced. After thorough medullary lavage, standard second-generation
techniques are used to insert antibiotic-impregnated cement. The
prosthesis should be cemented in 20 to 30 degrees of retroversion. This
can be determined by placing the forearm in neutral rotation, with the
transepicondylar axis of the elbow perpendicular to the trunk. The
humeral head should face the glenoid with 20 to 30 degrees of
retroversion in this position. The fin of the prosthesis of most
implants is then located just posterior to the posterior lip of the
bicipital groove. Excessive retroversion should be avoided to reduce
the tension on the greater tuberosity repair and reduce the risk of
secondary redisplacement (Fig. 35-33).
proximal shaft of the humerus, using the previously inserted
nonabsorbable sutures. The sutures may be passed through the holes in
the prosthesis to act as a stable fixation point, although some
surgeons prefer to attach the tuberosities directly to each other. The
sutures are assembled such that the intertubercular sutures are
orthogonal to the sutures that attach the tuberosities to the shaft. A
circumferential suturing technique, passing round the medial prosthetic
neck, has also been described.82,83,84
Some surgeons perform bone grafting in the area of the tuberosities, or
into the fenestrations of the newer prosthetic designs, using
cancellous bone from the excised humeral head. Newer fracture stems
also have a narrower metaphyseal segment and are hydroxyapatite coated,
in an attempt to enhance fixation of the tuberosities to the implant.
The high rate of tuberosity pull-off and nonunion has also generated
renewed interest in other enhanced suturing techniques to try to
encourage tuberosity healing.2,160
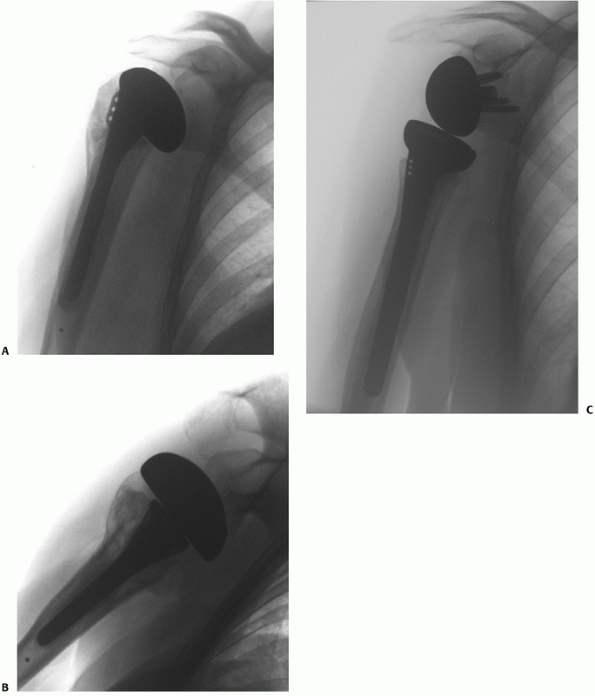 |
|
FIGURE 35-33
Nonreconstructable fractures may be treated with a humeral head arthroplasty. A conventional hemiarthroplasty may be associated with satisfactory shoulder function if the tuberosities heal, as shown in this case on anteroposterior and modified axial views (A,B). Reverse shoulder arthroplasty joint is a newer technique, which does not rely on the integrity of the rotator cuff for function. The results of the use of this technique for acute fractures are largely unknown at present (C). |
As an alternative strategy, the use of reverse total shoulder
arthroplasty has also been recently advocated. This lateralizes the
shoulder joint center of rotation, improving the mechanical advantage
of the deltoid and compensating for rotator cuff deficiency. This is
achieved by converting the glenoid into a spherical head and the head
of the humerus into a socket, thus providing a stable fulcrum for cuff
deficient shoulders (Fig. 35-33). The chief
theoretical advantage of the technique is that tuberosity healing may
not be as important in determining the functional outcome, because the
prosthesis is designed to function in cuff-deficient shoulders.
This may be acceptable for an elderly frail patient but is frequently
suboptimal in a younger, more active individual. It is notable that
despite extensive product marketing, there is no evidence to suggest
that the newer range of trauma arthroplasty implants produce a better
functional outcome than the generic implants.189
redisplacement or nonunion is probably the major reason for the
unsatisfactory functional outcome (Fig. 35-34).
Many patients are also unable to comply with an aggressive
rehabilitation program after surgery, and this may result in the
development of symptomatic periarticular adhesions and capsulitis.
|
TABLE
35-11 Summary of the Case-Series Results of Humeral Head Replacement Techniques for Proximal Humeral Fractures in Series That Report Functional Outcome* |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|
FIGURE 35-34 Tuberosity pull-off (arrow)
is a frequent complication of conventional arthroplasty for acute fracture. This complication is usually associated with a poor functional outcome. |
arthroplasty include instability, dislocation, prosthetic loosening,
late infection, and periprosthetic fracture. Most patients will not
survive long enough to develop these complications; those who do should
be treated as for a primary arthroplasty for degenerative joint disease.36,230,311
Periprosthetic fractures are difficult to treat and the risk of this
complication is increased in elderly, osteoporotic patients, who are
unsteady on their feet and prone to further falls.307 Nonoperative brace treatment may be used in frail patients,147
whereas open reduction and plate fixation is required in younger and
fitter patients, with a stable prosthesis. Revision to a long-stem
prosthesis may be required if there is substantial prosthetic loosening.312
with reliably good results, and the poor functional outcome associated
with traditional arthroplasty has led to its use being extended to
acute three- and four-part fractures and fracture-dislocations.
Encouraging early results have been reported using this technique in
case series (Table 35-11), but there is
currently a requirement for a comparative study with traditional
arthroplasty implants to assess the relative merits of these two
techniques. Reverse shoulder arthroplasty is associated with the same
risk of complications as conventional arthroplasty. In addition,
reverse arthroplasty is frequently complicated by notching of the
scapular neck, which may occasionally result in scapular stress
fracture. The longer-term risks associated with this complication are
as yet unknown.
to wear a shoulder immobilizer sling for 4 to 6 weeks. The period of
immobilization depends on the stability of fixation after
head-conserving techniques. Some surgeons prefer to rigidly immobilize
the arm for the first 4 weeks after an arthroplasty procedure, to
minimize the risk of tuberosity pull-off. Most surgeons prefer the
patient to commence pendular exercises and elbow range of movement
exercises immediately, with active-assisted range of motion exercises
commencing at 2 weeks after the operation. Abduction of the shoulder
beyond 90 degrees or external rotation beyond the neutral position is
prohibited during this period.
range-of-motion exercises supervised by a physiotherapist, supplemented
by a home exercise program, are commenced after removal of the sling
and continued for at least 6 months after the operation.
nonoperative treatment for the majority of patients with proximal
humeral fractures. The functional outcome in these patients will
usually be sufficient to their needs, and the risk of complications is
low. They are typically physiologically old, frail, and unable to
comply with an aggressive shoulder rehabilitation program and have a
fracture of a simple and stable configuration.
believes that there is no incontrovertible evidence on which to guide a
decision regarding the best management for the remaining minority of
patients.21,112,197
They are often younger and well motivated but have more complex
injuries. An individualized approach is adopted for these patients,
taking into account the patient-, injury-, and surgeon-related factors
that have been discussed previously. Operative treatment is considered
when the individual patient’s functional outcome is likely to be
significantly improved by the surgery or when nonoperative treatment
would be associated with an unacceptably high risk of complications or
severe functional impairment. The most common complications that give
rise to this degree of functional disability are nonunion,
osteonecrosis, and rotator cuff dysfunction. Although rare, any one of
these complications will usually compromise the functional outcome for
the patient. In contrast, if a fracture heals with a perfused head and
a functional rotator cuff, the patient will usually have good shoulder
function, regardless of the radiologic appearance.
falls into two categories with distinctly different functional
outcomes: Humeral head-conserving procedures should aim to anatomically
reduce and fix the fracture in a stable manner. If this subsequently
heals without osteonecrosis and with a functional rotator cuff, the
patient will have a good functional outcome. This form of treatment
should therefore
be
regarded as an “opportunity” for the patient to regain a pain-free,
functional shoulder. The level of function may approach the preinjury
state in younger, well-motivated individuals. In contrast, most of the
contemporary literature suggests that the use of humeral head
arthroplasty is associated with a pain-free but poorly functional
shoulder, because of tuberosity pull-off or rotator cuff dysfunction
and failure.
should be the default primary operative treatment for the majority of
complex proximal humeral fractures, where the humeral head appears
viable and can be reconstructed. Primary arthroplasty is reserved for
the minority of patients, where nonoperative treatment is likely to be
associated with a high risk of complications, and a humeral
head-conserving procedure is infeasible. The latter situation only
arises if the humeral head is devoid of soft tissue attachments and has
a high risk of osteonecrosis, or rarely when the fracture is
technically unreconstructable. Therefore, if a surgical reconstruction
is contemplated, it is the author’s routine practice to evaluate the
detailed anatomy of the fracture on spiral CT scanner with
three-dimensional reconstructions, before surgery. In this way, a more
informed decision can be made about whether the humeral head is likely
to be salvageable at surgery. This allows better preoperative
counseling about the likely form of surgical reconstruction and
eventual outcome.
techniques, as it is believed that superior anatomic fracture reduction
and stable fixation can be better achieved using open techniques (Table 35-12).
The extended deltoid-splitting approach, through a shoulder-strap skin
incision, is used in almost all cases. The approach is especially
useful for of Type I anterior fracture-dislocations73,252 and all posterior fracture-dislocations.244,245
In Type II anterior fracture-dislocations, the humeral head is usually
denuded of all soft tissue attachments and may be very mobile and
difficult to retrieve into the wound through the deltoid split. In
these circumstances, a separate limited deltopectoral approach is
sometimes required, through the anterior portion of the shoulder strap
incision to retrieve the humeral head. The only fracture in which a
deltopectoral approach is used primarily is the two-part displaced
lesser tuberosity fracture, where better access is provided through a
direct anterior approach.257 All humeral head arthroplasty procedures are also performed through a deltoid-splitting incision.
|
TABLE 35-12 Goal-Orientated Treatment of Proximal Humeral Fractures
|
|||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||
described previously. Routine radiologic screening of the fracture is
undertaken at 1 to 2 weeks to ensure secondary displacement has not
occurred, especially if there are multiple undisplaced fracture lines
or if the shaft is not fully impacted on the humeral head and is
thereby potentially unstable. Elbow, wrist, and hand mobilization
begins immediately, with shoulder pendular exercises beginning when
there is radiologic confirmation of maintenance of fracture reduction.
3 weeks, progressing to isotonic strengthening and stretching exercises
at 6 to 12 weeks following injury. The patient is advised to continue
shoulder exercises during the first year after injury, because delayed
restoration of movement may continue during this time. Emphasis is
placed on a patient-orientated home exercise program, rather than
prolonged supervised physiotherapy. Outpatient monitoring continues
until there is evidence of fracture union both clinically (absence of
pain, restoration of function and range of motion) and radiologically.
Prolonged radiologic reassessment thereafter is not logistically
feasible in our unit, with the large numbers of patients treated.
However, we advise patients to return for reassessment if they notice
increased pain or worsening function in their shoulder.
initially treated nonoperatively, as for other one-part fractures.
However, repeat
radiographs
are obtained at 1 and 2 weeks postinjury, as secondary displacement may
occur through the pull of the attached supraspinatus and infraspinatus
tendons. In physiologically older and frail patients (usually older
than 80 years) with limited functional expectations, a substantial
degree of displacement is tolerated without recourse to operative
treatment. These patients often have a poor outcome with attempted
surgical reduction and fixation, because of their poor bone quality and
pre-existing cuff dysfunction, which precludes stable fixation.
Although they will often have signs of continued cuff dysfunction from
the tuberosity nonunion or malunion, their functional outcome will
usually be adequate for their needs.
younger (typically younger than 65 years), active patients with
fractures, which are either primarily displaced by more than 5 mm or
become displaced by this amount within the first 2 weeks postinjury. In
addition, selected older patients (typically aged between 65 and 80
years) with fragment displacement of 1 cm or more are offered operative
reconstruction. When there is a substantial tuberosity fragment
(greater than 2.5 cm), open reduction through a limited
deltoid-splitting approach and internal fixation using partially thread
cancellous 3.5-mm screws are performed. It is important to insert
screws to transfix the fragment to both the humeral head and the medial
cortex of the metaphysis. Meticulous repair of any associated rotator
cuff injury is also performed.
heavily comminuted, it is the author’s policy to treat these injuries
in the same manner as a rotator cuff avulsion. Arthroscopic assessment
is performed and, if feasible, the small tuberosity fragments are
repaired to their native bed, using a combination of cuff and
interosseous sutures attached to suture anchors inserted at the margins
of the cuff avulsion. As with all arthroscopic rotator cuff repairs, a
“double-row” fixation to the “footprint” of the greater tuberosity is
preferable to reduce the risk of later repair failure and to maximize
the healing potential.38,171
If arthroscopic repair is not possible, the tuberosity is reconstructed
through a limited deltoid-splitting approach using a similar technique
of transosseous repair.81
tuberosity fracture is associated with a bony glenoid rim avulsion is
often associated with acute instability of the shoulder.250 For this reason, the author routinely treats both of these injuries with primary internal fixation.
younger or middle-aged patients and are displaced. Nonoperative
treatment of these injuries risks later functional incapacity, because
of subscapularis dysfunction. It is the author’s policy to treat all
these fractures operatively in medically fit patients. ORIF is
performed through a standard deltopectoral approach. If there is a
single large fragment, definitive internal fixation is performed using
partially threaded 3.5-mm cancellous screws, inserted through the
lesser tuberosity.257 Judging
accurate screw length (typically between 40 and 50 mm) is an important
technical aspect of the procedure, to gain bicortical purchase. Where
the fragment is 2.5 cm or less or if there is comminution, screw
fixation risks secondary comminution or does not give sufficient
stability. For these patients, the reduction is maintained by
interosseous sutures, placed both through the tuberosity and through
the bone-tendon junction, which are tied to the adjacent metaphyseal
bone.257
fracture is associated with a locked posterior dislocation, an attempt
is initially made to obtain closed reduction of the dislocation under
anesthesia. Where this is not possible, an open reduction is performed
through an extended deltoid-splitting approach. After reduction of the
shoulder has been obtained, the stability of the shoulder is assessed
throughout a full range of internal and external rotation movement,
both with the arm at the side and in 90 degrees of abduction. If the
shoulder is acutely unstable because of reengagement of a reverse
Hill-Sachs lesion on the posterior glenoid beyond neutral rotation, the
reverse Hill-Sachs lesion is either elevated and bone-grafted or, if
there is a larger defect, filled with a sculpted femoral head
allograft, which is secured with two countersunk 3.5-mm partially
threaded cancellous screws. The lesser tuberosity is then reattached
anatomically, using the same techniques as for other isolated two-part
fractures, using either two 3.5-mm partially threaded cancellous screws
or interosseous sutures.
the surgical neck are treated nonoperatively. A substantial degree of
translation of these two fragments is usually tolerated, as long as
there is residual cortical contact and impaction. Occasionally, if
there is severe varus angulation of the head fragment in a
physiologically younger individual (typically younger than 65 years),
operative disimpaction, anatomic reduction, and plate fixation will be
performed to reduce the risk of later impingement of the greater
tuberosity in the narrowed subacromial space and dysfunction of the
rotator cuff from its shortened lever arm.
comminuted surgical neck fractures, where there is no appreciable
contact of the humeral head with the shaft, are mechanically unstable
and therefore prone to nonunion. In physiologically younger patients
(typically aged under 65 years), open reduction and fixation is
performed, using either a standard clover leaf or locking plate,
dependent on the bone quality in the humeral head. Unfortunately, many
of the patients who sustain these unstable injuries are either elderly,
frail individuals (typically older than 80 years), or younger but with
multiple medical comorbidities or alcohol-dependency problems. This
type of injury is therefore often a “marker” of their poor physiologic
status. These patients are still at a higher risk of nonunion, but
operative treatment may be contraindicated because of either their high
anesthetic risk or the likelihood of operative fixation failure.
Although these patients are often treated nonoperatively, operative
fixation should be offered if at all possible. This is because nonunion
is almost always associated with pain and severe functional disability
and operative treatment of the primary injury is always technically
easier than the treatment of an established nonunion.
wherever possible. It is important to restore continuity of the medial
calcar support to prevent acute fixation failure using either
structural graft or a low inferomedial screw, which is inserted
separate from the plate. Occasionally, if there is extensive
metaphyseal
comminution
in an older patient, the risk of nonunion across the area of
comminution is high. In these circumstances, shortening and impaction
of the shaft fragment within the head is performed to produce a more
stable configuration before plate application. If the bone quality in
the humeral head is very poor, a short proximal humeral locked
intramedullary nail will often provide more secure fixation than a
plate. However, a locking plate is preferred for definitive fixation,
if at all possible, to minimize the risk of the later rotator cuff
dysfunction, which nailing usually produces.
should be treated nonoperatively, if there is residual cortical
continuity of the humeral head fragment on the shaft, the tuberosities
are not too widely displaced, and humeral head appears viable. Although
the outcome is often imperfect, after union these patients will usually
have a pain-free shoulder, which has sufficient function for their
everyday needs.
younger patients, where it is thought that the risk of nonunion, cuff
dysfunction or osteonecrosis is high or where operative treatment is
likely to provide a significant improvement in shoulder function over
nonoperative treatment. In practice, this means that surgery to prevent
nonunion or cuff dysfunction is often offered to patients with
fractures in which the humeral shaft and tuberosities have
significantly displaced from the humeral head. The risk of
osteonecrosis is determined by the fracture configuration, with wide
displacement of the head from the shaft with likely loss of the medial
periosteal and capsular hinge, and the absence of a medial metaphyseal
spike particularly associated with a higher risk of this complication.
There are substantial functional gains from internal fixation for
fractures in which the humeral head has displaced from its normal 130
degree head-shaft orientation to occupy an extreme position of varus
(90-degree head-shaft angle) or valgus (180-degree head-shaft angle) or
where there is marked humeral head articular surface incongruity from
displaced marginal articular fragments attached to the tuberosities.
can provide an indication of the likelihood that this will be feasible.
Operative technique follows the protocol previously described: The goal
is to attempt anatomic or near-anatomic reconstruction, and the author
freely uses bone substitutes and allograft to fill metaphyseal defects
and supplementary screws to reduce and fix articular surface fragments
that are attached to the tuberosities. Definitive internal fixation is
performed, using either a cloverleaf or proximal humeral locking plate,
dependent on the quality of the bone in the humeral head.
the fracture is deemed to be unreconstructable, a cemented humeral head
hemiarthroplasty will be performed. This is usually only indicated if
the head is devoid of soft tissue attachments and shows no back
bleeding. The patient is counseled that the likely outcome after an
arthroplasty will be a pain-free but stiff shoulder. However, an
arthroplasty is more likely to function well when it is performed as a
primary, rather than as a salvage procedure.
for all these fractures, as their risk of osteonecrosis is much lower.
A closed manipulation of this type of injury should never be attempted,
because of the risk of dislodging the head and damaging residual soft
tissue attachments.121,252
Through an extended deltoid-splitting approach, the fractured
tuberosities are tagged with stay sutures and gentle traction allows
exposure of the anterior glenoid margin to reveal the dislocated
humeral head. Insertion of an osteotome or a blunt elevator between the
anterior glenoid rim and the engaged Hill-Sachs lesion of the humeral
head is required to disengage the two structures, after which reduction
of the humeral head can be achieved, by translating it forward to face
the glenoid. Following its relocation, the humeral head usually has
retained anterior and inferior capsular attachments, but active
arterial bleeding from drill holes inserted into the raw cancellous
surface of the back of the humeral head confirms active perfusion. The
fractures are then anatomically reconstructed using suturing of the
tuberosities, locking-plate fixation, with filling of any metaphyseal
defects using allograft or bone substitutes as described previously.
Operative treatment is not required for either associated bony or soft
tissue labral pathology, or the posterior humeral head defect, as
anatomic reduction and fixation of the fracture usually restores full
shoulder stability.
injuries, the humeral head is almost inevitably devoid of soft tissue
attachments and there is no evidence of active arterial bleeding from
the raw cancellous surface of the back of the humeral head following
relocation. A head-salvaging reconstruction with plate fixation is
therefore less likely to be successful, because of the risk of later
osteonecrosis. It may be worth attempting this in a physiologically
younger patient (typically younger than 65 years), accepting that the
risk that a later humeral head arthroplasty may be required.
treatment rests between closed manipulation and humeral head
arthroplasty. It is the author’s policy to initially attempt a gentle
closed manipulation of the shoulder under general anesthesia. Minimal
traction is required, and instead an attempt is made to maneuver the
head through the rent in the inferior capsule to achieve relocation
against the glenoid. There is a substantial risk of disengaging the
humeral head from the shaft,121,252
but if a closed relocation of the head can be achieved, the injury is
then usually stable and can be treated nonoperatively. The use of
temporary percutaneous pinning of the dislocated head to the shaft may
prevent their disengagement.121 As
with younger patients, there is a substantial risk of later
osteonecrosis following a successful relocation, but many of these
frail elderly patients may not develop these symptoms within their
limited life span. If a closed relocation cannot be achieved or if the
head becomes disengaged from the shaft, a standard cemented
hemiarthroplasty is performed
anterior-fracture dislocations, it is the author’s policy to attempt
ORIF of all these fractures, as their risk of osteonecrosis is low. A
similar operative approach is used: The engaged humeral head is
disimpacted from the posterior glenoid rim with a bone lever.
Relocation
is then achieved under direct vision by rotating the humeral head
forward to face the glenoid. The reduced humeral head is almost
invariably perfused, with residual capsular attachments and active
arterial back-bleeding from its fractured cancellous surface.
stabilized using two or three Kirschner wires to restore rotational
stability and allow assessment of residual posterior instability.
Judging the accuracy of the reduction of the primary fracture is often
difficult, where marginal impaction has produced an acute osteochondral
fracture of the humeral head (reverse Hill-Sachs lesion). However, the
presence of the intact posterior capsule and periosteal “hinge” is a
useful aid to reduction, serving as a template for “closing the door”
to achieve the reduction.
(reverse Hill-Sachs lesions) that persist after provisional reduction
of the head and that are engaging the posterior glenoid rim with the
shoulder in neutral rotation are considered to be a potential source of
posterior instability. Where there is a large residual osteochondal
fragment with retained soft tissue attachment, this is treated by
elevation of the osteochondral fragment, with insertion of morselized
femoral head allograft to buttress the elevated fragment and support
from “positional screws.” Where the fragments are larger, comminuted,
or loose, a deep-frozen femoral head osteochondral allograft is
sculpted on-table to fill the defect. These procedures usually restore
full on-table stability, and repair of posterior soft tissue labral or
bony glenoid rim fractures is seldom required.
configuration. Two-part anatomic neck fractures are stabilized using
3.5-mm partially threaded cancellous lag screws. These are inserted
perpendicular to the fracture line into the humeral head, through the
intact lateral proximal humeral metaphysis. The humeral head component
of the more complex three- and four-part fractures is fixed to the
tuberosities, using either lag screws or interosseous sutures. This
composite is then stabilized to the humeral shaft using either a
cloverleaf or locking plate, dependent on the bone quality.
modified slightly, and a foam pad or pillow is used to restrict the
patient from internally rotating their shoulder beyond a neutral
position for 4 weeks, to protect against potential posterior
instability.
but more severe injuries are usually seen after industrial or heavy
machinery accidents, motor vehicle accidents, or gunshot/blast
injuries. They are characterized by the severity of the soft tissue and
bony injury to the shoulder, which is often associated with a
neurovascular injury. They may occur in association with multiple
injuries to other systems, as well as other orthopaedic injuries.
guidelines, with life-threatening injuries managed first, before
attention is turned to the shoulder injury. Any vascular injury to the
shoulder takes priority and should be repaired immediately if there is
acute ischemia. Vascular reconstruction may be contraindicated if the
distal circulation is good through the collateral circulation, without
an expanding hematoma, and there is a complete plexus avulsion, which
is unreconstructable.
as for any other open fracture. Any dead or devitalized bone should be
removed. Topical antibiotic beads and parenteral antibiotic therapy are
provided, and repeated wound inspection and redébridement and soft
tissue coverage with local flaps may be required. If there is more
extensive fracture comminution or bone loss, temporary splintage and
maintenance of length and alignment may be possible using temporary
external fixation or fine wire fixation until soft tissue cover has
been obtained. Delayed staged reconstruction can then be performed,
using either internal fixation and bone grafting or Ilizarov
techniques, dependent on the fracture configuration.
approximately 2 to 3 months, but it depends on the nerves involved, the
type of injury, and the age of the patient. Nerve recovery is better
with younger patients and more distal injury. The recovery from a sharp
injury is better than from a blunt injury. Early exploration and repair
are usually indicated after a penetrating injury with complete motor
loss in a given motor group.
multiple injuries, and internal fixation of the fracture is frequently
contemplated as part of early total care. However, these patients often
have lower limb injuries that require them to use crutches. They
therefore use their upper limbs more than normal during transfer and
mobilization, and this can result in early fixation failure of the
proximal humeral fracture. This is because these patients are placing
nonphysiologic cyclic loads through their arm during weight-bearing.
Early internal fixation of the proximal humeral fracture is therefore
only recommended if the patient is able to rest his or her arm in a
sling during subsequent rehabilitation.
Extension of a proximal humeral fracture into the shaft is occasionally
encountered, through either a long medial metaphyseal spike, extensive
metaphyseodiaphyseal comminution, or a true segmental fracture. The
proximal humeral fracture is commonly, but not invariably,
extra-articular, with minimal tuberosity involvement. These uncommon
injuries are unstable, and attempted nonoperative treatment is
difficult since adequate splintage cannot be provided. There is a high
risk of nonunion and late reconstructive surgery is complex because of
the extensive fibrous union and the complex three-dimensional deformity.
injuries, but there is a substantial risk of rotator cuff dysfunction
at the shoulder. Although the technique of helical plating has been
used,94,314
the early treatment of these injuries has recently been greatly
simplified by the provision of longer proximal humeral locking plates,
which allow bridging of comminuted areas and rigid fixation across
segmental fractures (Fig. 35-35). This can be
accomplished either through distal extension of a deltopectoral
approach into an anterolateral approach to the humerus or through an
extended deltoid-splitting approach. The deltoid insertion must be
split to accommodate the longer plate, but this can be repaired after
plate application without deleterious effects.
sustain their injury,57
and most are treated nonoperatively as outpatients. Despite this, 1 in
10 patients will have died within the first year after sustaining this
injury, and 1 in 3 have died by 5 years.220,221,222,274
The overall mortality in the first year is more than three times
greater than that of the general population, and twice as great in the
subsequent 4 years.274
Paradoxically, although the highest mortality rate is seen in elderly
patients following proximal humeral fracture, the risk of death is
higher in younger individuals compared with the general population.
Many of the patients who sustain this injury at a younger age have risk
factors for the development of premature osteoporosis including, most
commonly, premature menopause, arthritides, immobility, malignancy,
prolonged drug treatment (particularly steroid and anticonvulsant
medication), medical comorbidities, excessive tobacco use, excessive
alcohol ingestion, and neuromuscular disorders.274,220,221,222 These prefracture
comorbidities reflect a reduced physiological reserve in these younger
patients, and this may be responsible for their curtailed survival,
compared with their peers in the general population. Medical
optimization of these comorbidities should be a key priority in the
treatment of younger individuals who sustain low-energy fractures,
especially if they require surgery to treat their fracture.
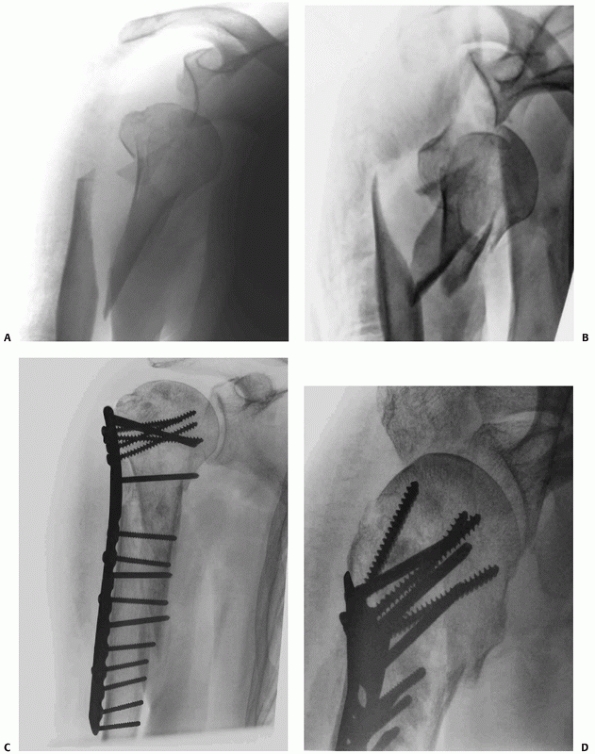 |
|
FIGURE 35-35
Fractures that extend into the diaphysis can be treated by the use of a long locking plate. In this fracture, there was also a dislocation of the humeral head with a significant humeral head-split (A,B). Following reduction and fixation with a long locking plate, the fracture healed without signs of osteonecrosis at 2 years (C,D). |
as a result of pain, loss of use of the limb, and reduced mobility,
from difficulty in using walking aids. These patients are also at
substantially greater risk of sustaining other osteoporotic fractures,256
and careful consideration should be given to secondary preventative
treatment to reduce this risk after the index proximal humeral fracture.
fractures that have been described, but most are rare. Complications
may occur as an inevitable consequence of a more severe injury or as a
result of treatment. The latter may be because of errors in procedure
selection or in the surgery provided. Implant-specific operative
complications have been described in the previous sections and will not
be discussed further here.
these fractures, for those patients receiving delayed treatment using
an arthroplasty (Table 35-13). Some postinjury complications are not considered in this classification,26
although it does serve to highlight the three most common sources of
complications (osteonecrosis, nonunion, and tuberosity malunion causing
cuff dysfunction), which may compromise the functional recovery after
this injury, as well as highlighting the poor prognosis associated with
arthroplasty procedures in which osteotomy of the greater tuberosity is
required. These three complications are considered in detail, together
with an overview of the range of other complications and their
treatment, as follows.
consequence of loss of perfusion of the articular surface and
subchondral bone, which undergo involutional change, leading to
articular collapse and fibrosis. This condition may or may not be
symptomatic,305 and the head may collapse completely, or there may be partial involvement, with or without articular collapse.14,123
The spectrum of presentation, the lack of precise radiological
guidelines for its assessment, and the wide range of treatments make
estimation of its prevalence difficult (Tables 35-6, 35-8, 35-9, 35-10, and 35-11).
|
TABLE 35-13 Boileau et al.’s Classification of Complications of Proximal Humeral Fractures and Their Recommended Treatment
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
the injury, because of the irrevocable injury to the head blood supply.
Three- and four-part fractures and fracture-dislocations are therefore
at higher risk than are one- and two-part fractures. It may also occur
as a consequence of operative treatment, because of excessive fracture
manipulation and stripping of soft tissues, which contain the residual
vascularity to the articular segment. Some individuals may also be
predisposed to this complication, either through their poor physiologic
state, through their medical comorbidities and drug treatment, or
through smoking and alcohol abuse. The pathophysiology of this
condition is incompletely understood at present and other unknown
factors may also be important. It does not invariably develop even if
the head is completely denuded of blood supply,14,103,123 whereas some cases appear to occur “sporadically” after a relatively innocuous injury.
loss of function, typically after a “latent” period, where function has
been satisfactory. Radiologically, the changes vary from patchy and
segmental humeral head sclerosis, to complete humeral head resorption
and collapse (Fig. 35-36). The differential
diagnosis is from posttraumatic osteoarthrosis, in which the degree of
collapse is usually less severe, and from chronic joint sepsis, which,
if clinically suspected, should be excluded by bacteriologic
examination of a joint aspirate. CT and especially MRI are useful in
the evaluation of the extent and severity of head involvement.
If there are reciprocal glenoid changes, a total joint arthroplasty may
be more successful in relieving pain and restoring motion.68
Where there is a severe associated tuberosity malunion or cuff tear,
reverse shoulder arthroplasty may provide better function, although
comparative studies have not yet been performed to evaluate this.
Resorption, sclerosis, and collapse may be seen after fracture of the
greater tuberosity (the “disappearing tuberosity”) and may also occur
in the lesser tuberosity. It may occur after two-, three-, or four-part
fractures and may follow nonoperative treatment, internal fixation, or
arthroplasty. This complication is predictable, because until a
fractured tuberosity unites, it only receives blood supply through
residual periosteal attachments and its cuff attachments, which are
often relatively avascular in elderly patients. The patient usually has
debilitating shoulder pain and loss of function, with clinical signs of
rotator cuff weakness and dysfunction. Subclinical forms probably occur
frequently, and the precise pathology has not yet been fully
elucidated. At present, there is no known treatment for this
complication.
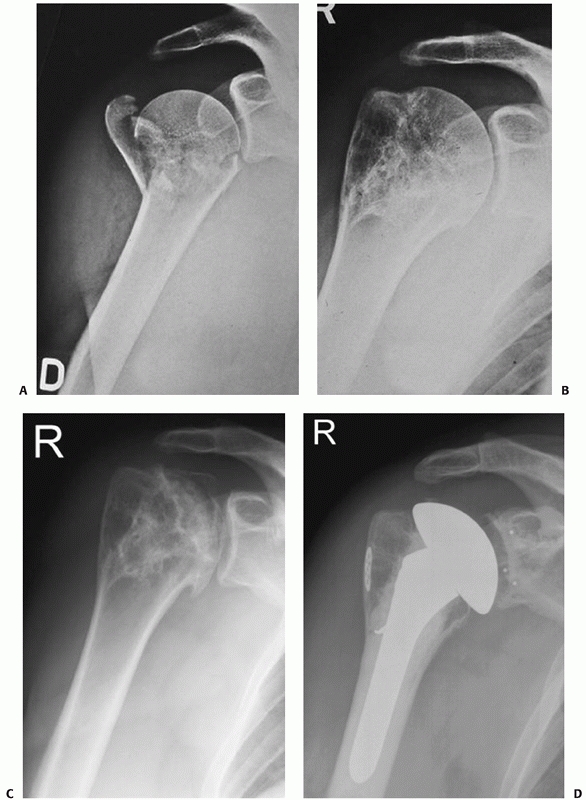 |
|
FIGURE 35-36
Osteonecrosis may occur after both nonoperative and operative treatment and is normally treated using a conventional hemiarthroplasty (A-D). (continues) |
The normal time for clinical union of a proximal humeral fracture is
typically 4 to 8 weeks. It is therefore logical to define nonunion to
be present if a fracture site is still mobile 16 weeks postinjury,
although 6 months has been used in some studies. In the only study to
have evaluated the epidemiology of this complication, the overall
reported
incidence was 1.1%, although this rose to 8% if metaphyseal comminution
was present and 10% if there was displacement of the surgical neck.60
Nonunion of one or both tuberosities to the head is less common and is
considered together with tuberosity malunions later section below.
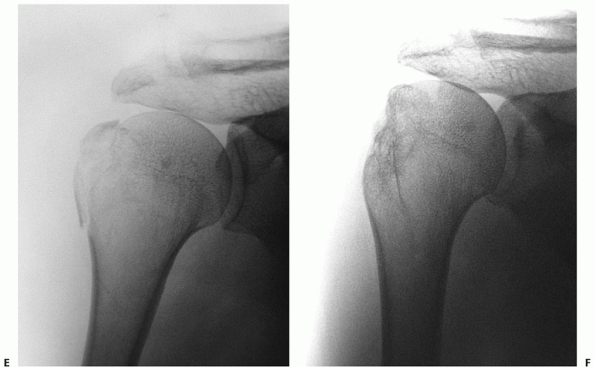 |
|
FIGURE 35-36 (continued) Osteonecrosis of the greater tuberosity (the “disappearing tuberosity”) is also encountered, and is not amenable to treatment (E and F).
|
instances there are identifiable patient-, fracture-, or
treatment-related risk factors: Common patient-related factors include
osteoporosis, poor physiologic state, medical comorbidities and drug
treatment, heavy smoking, and alcohol abuse.118,270,300 Shoulder inflammatory or degenerative joint disease, causing preinjury shoulder stiffness, may also predispose to nonunion.258
The fractures most at risk of nonunion are two-, three-, or four-part
displaced fractures, where there is no residual cortical contact
between the humeral head and shaft, and those where there is marked
metaphyseal comminution.60 The
complete disruption of periosteal sleeve leads to mechanical
instability, and soft tissue interposition of periosteum, muscle, and
the tendinous portion of the long head of biceps may also inhibit
callus formation.87,205
During nonoperative treatment, it is stated that the use of hanging
casts, which distract the fracture, and overzealous shoulder
mobilization may predispose to nonunion.209
Poor surgical technique with extensive soft tissue stripping and a
mechanically unstable fracture reduction and fixation may also
predispose to nonunion.
seldom a problem. Pain, stiffness, and loss of function in the arm are
the most constant complaints. The pain tends to be severe and
debilitating and is aggravated by use of the arm and shoulder, because
most movement occurs at the site of the nonunion. This is not amenable
to splinting, and use of a sling further compromises shoulder function.
On examination, the patient often has a “pseudoparalysis” of the
deltoid, rotator cuff, and periscapular muscles, with a flail arm.
Attempted movement of the shoulder is painful and any motion occurs in
the fracture site rather than the glenohumeral joint. Radiologically,
there is resorption and widening of the fracture line, often with
massive bone resorption (Fig. 35-37).
nonunion and assess the state of the humeral head articular cartilage,
the degree of separation and healing of any associated tuberosity
fractures, and the feasibility of reduction and fixation of the
fracture. If surgical reconstruction has been previously performed,
infection should be excluded at the site of the nonunion by culture of
an aspirate performed under ultrasound control.68
the development of this complication can only be provided by operative
treatment. This is technically challenging, because of capsular
contractures and scarring from previous surgery, bone loss, distorted
anatomy, and osteopenia of the humeral head.87,88
Unfortunately, many patients with established nonunions are too
elderly, frail, or medically unfit to undergo this type of surgery and
the prolonged shoulder rehabilitation program thereafter. Pain
management and activity modification are all that can be offered to
these patients.
reconstruction. Attempts have been made to classify these according to
their anatomic site and the degree of bone loss.44
In practice, the chief decision is whether the nonunion is amenable to
an attempt at ORIF or whether a humeral head procedure is required. The
decision as to which form of treatment is most appropriate is
individualized, but absence of infection, adequate humeral head bone
stock, lack of severe tuberosity malunion, and absence of degenerative
change or collapse of the articular surface are mandatory for an
attempted open reduction and plate fixation.
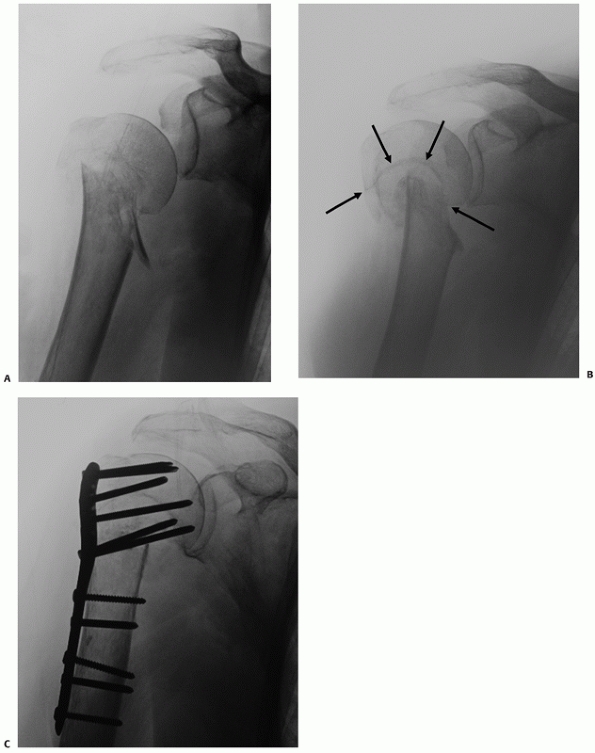 |
|
FIGURE 35-37 Nonunion after nonoperative treatment of a translated surgical neck fracture (A) at 3 months (B)
is a debilitating complication. There is massive metaphyseal bone loss from the “windshield-wiper” effect of the shaft in the head (arrow). Reduction and internal fixation was achieved by excision of the nonunion and shortening to bayonet the shaft fragment into the humeral head (C). |
but since this technique provides less rigid fixation at the site of
the nonunion, the technique is less commonly used than open reduction
and plate fixation. The exposure is similar to that used for primary
plate fixation. The nonunion is exposed and taken down by excision of
the fibrous union and pseudocapsule and removal of any devitalized bone
fragments. Arthrolysis of a stiff joint may be required, which aids in
subsequent rehabilitation and limits force transmission at the nonunion
site.118 However, care must be taken
to avoid devitalization by excessive soft tissue stripping to expose
the nonunion. It is essential to ensure that the bone ends are bleeding
and the medullary canal is clear of fibrous debris.
in a relatively anatomic fashion. This is unusual, and more commonly
there is extensive metaphyseal bone loss because of the
“windscreen-wiper” effect of the shaft at the site of the mobile
synovial nonunion. Satisfactory viable bone contact can usually only be
achieved by impaling the relatively narrow bayonet of the shaft into
the sheath of the wide humeral head metaphysis. The nonunion site is
typically autografted with either corticocancellous strips of iliac
crest bone graft, an intramedullary bone plug, or a fibular strut graft.301
This may result in significant humeral shortening, but this is usually
well tolerated. If maintenance of length is deemed to be important in a
younger patient, this requires more extensive grafting of the
metaphyseal defect, using autogenous bone, augmented with fibular strut
grafts.
definitive plate fixation is then performed. A locking proximal humeral
plate is the ideal implant in this situation, given the relatively poor
proximal humeral bone stock. Postoperative rehabilitation follows the
standard guidelines as for primary fixation.
depends on whether they are healed and their degree of displacement.
Ununited fragments may be amenable to reduction and stabilization using
the osteosuturing techniques previously described, whereas healed,
minimally displaced tuberosity fragments do not require adjuvant
treatment. Tuberosity osteotomy may occasionally be used if there is a
severe malunion, but this should be avoided if at all possible because
of the risk of subsequent nonunion of the fragment.
reconstruction of established nonunions have reported a high success
rate in achieving union, often with a good eventual functional outcome.*
However, a high rate of postoperative complications is to be expected,
which may require further surgery, and the functional recovery time is
usually prolonged.
head and metaphyseal bone stock and cavitation, severe tuberosity
malunion or displaced nonunion, or collapse and degenerative change of
the humeral articular surface. The main aim of treatment is pain
relief, and the eventual poor functional recovery is often worse than
for a primary arthroplasty. Although hemiarthoplasty is most commonly
used, total shoulder replacement may be indicated if there is glenoid
articular surface wear or defects. The functional outcome is poor when
osteotomy of the tuberosities is performed, and this should be avoided
if possible.26,27
It has been suggested that reverse shoulder arthroplasty may improve
the shoulder function in patients with nonunions associated with severe
tuberosity malunions,26 although comparative studies have not yet been reported.
arthroplasty to treat nonunion suggest that the procedure may be
effective in reducing or eliminating pain but with a high rate of
complications, often requiring further surgery and a disappointing
functional recovery.5,26,118,205,214
It remains to be seen in future clinical studies whether the use of
reverse shoulder arthroplasty will improve the outcome over
conventional arthroplasty in the treatment of this challenging
complication.30 At present, an
arthroplasty should only be considered for patients who have poorly
controlled pain and have nonunions that are not amenable to reduction
and internal fixation.
proximal humeral fractures that are treated nonoperatively. It may
occur after surgery through intraoperative malreduction or through
inadequate fixation that allows secondary redisplacement. Two types of
malunion are distinguishable and often coexist: malunion of the head on
the shaft either through impaction, translation, rotation, or
angulatory deformity is common and is well tolerated in most patients.
Malunion of one of both tuberosities is also common and well tolerated
in older patients with limited functional expectations. However, in
physiologically younger patients, the altered shoulder mechanics
produced by the defunctioning and tearing of the rotator cuff tendons
and mechanical impingement of the displaced tuberosity fragments often
produces an unacceptable degree of pain and functional compromise.
Where the two conditions coexist, it is therefore most often the
tuberosity malunion that is symptomatic.
shoulder pain, which is typically localized over the anterior deltoid.
The pain is typically aggravated by use of the arm, particularly in
forward flexion, abduction, and internal rotation movements. This
frequently results in impairment of the patient’s ability to perform
normal daily activities and leisure pursuits. It is important to try to
distinguish the cause of symptoms on physical examination, because
rotator cuff impingement and tears, posttraumatic shoulder stiffness,
acromioclavicular joint dysfunction, biceps tendinopathy, and complex
region pain syndromes may all be contributory to symptomatology. In
addition to specific clinical testing of these structures, a good
response to subacromial local anesthetic tends to localize symptoms to
the subacromial space. If infection is suspected, appropriate
hematologic studies and bacteriologic examination of a joint aspirate
are warranted.
appreciated using CT with three-dimensional reconstructions. MRI may be
useful in evaluating the state of the rotator cuff and capsule, but
interpretation of images is frequently hampered by the distorted
anatomy. It may be useful in detecting radiologically occult early
osteonecrosis. As with nonunion, attempts have been made to classify
this complication,17,26
but most often treatment is individualized based on the patient’s
physiologic status and level of symptoms, the anatomy of the injury,
and the likelihood of success from a surgical reconstructive procedure.
for older patients a trial of nonoperative treatment is advisable. A
shoulder rehabilitation program, pain management, and activity
modification may reduce the symptoms to acceptable levels and improve
function. The patients who remain symptomatic despite this treatment
and request surgery should be carefully counseled about the likely
limited gains from surgery, as well as the significant risk of
complications. The technical details of the operative treatment
according to the anatomic pattern of malunion are discussed next.
tuberosity fracture are relatively common but are usually debilitating
only in younger, physically active patients. The tuberosity retracts
posterosuperomedially by the deforming forces of its attached
cuff
muscles, but the articular surface is unaffected. The posterior
displacement may produce a bony block to external rotation, whilst
superior displacement may block abduction and lead to subacromial
impingement. Tuberosity malposition can also produce cuff dysfunction,
attrition, and tears.278
Arthroscopic assessment may provide useful extra information and may
occasionally be amenable to arthroscopic mobilization and fixation in
the presence of a relatively mobile nonunion.98
deltoid-splitting approach to gain access to the displaced tuberosity,
which is usually fixed and immobile. The fragment is mobilized by
excision of the fibrous nonunion or osteotomy of a malunion. An
extensive posterior capsular release or excision and a rotator interval
dissection are often required to mobilize the fragment sufficient for
it to be reduced to its decorticated native bed. Fixation is achieved
using either interosseous sutures or screw fixation, as for an acute
fracture. It is important to test the repair by fully internally
rotating the arm, to ensure the repair is not unduly tight. An
acromioplasty and subacromial decompression should be performed if the
subacromial space is narrowed, to reduce the risk of later impingement.
If the repair is tight, the arm is immobilized for 4 weeks in neutral
or slight external rotation, to reduce the risk of failure of the
repair. The postoperative treatment protocol is otherwise identical to
the treatment of an acute fracture. There are a few reports of the
results of treatment of greater tuberosity malunions,17 which report substantial pain relief and functional improvement but with prolonged recovery times.
The displaced fragment may block internal rotation or cause
subscapularis weakness, and occasionally this may be amenable to
arthroscopic treatment.126 The
fragment is exposed through a deltopectoral approach and mobilized with
capsular releases as for malunion of the greater tuberosity. ORIF with
anatomic reduction of associated articular involvement can be performed
with heavy interosseous sutures or screws, dependent on the fragment
size.
severe debility unless the humeral head heals with a varus deformity
sufficient to cause secondary cuff impingement or dysfunction.15,68,278,279,286
The deformity is characteristically a complex angulatory (varus or
rarely valgus) and internal rotational malunion, with translation of
the shaft anteromedially.17,68,278,279
Operative treatment is seldom indicated except in younger patients who
are still symptomatic after a prolonged shoulder rehabilitation
program. If clinically indicated, osteotomy to correct the deformity
and locking plate fixation are performed.15,286
Open capsular release is usually performed if there is significant
associated posttraumatic stiffness and the osteotomy is usually bone
grafted. The results from this technique are satisfactory in most
reported series.17,286
and the deformity is less severe, soft tissue releases and an osteotomy
of the fracture fragments, followed by internal fixation, may be
attempted. A successful outcome can only be achieved if there is a good
correction of both osseous and soft tissue abnormalities.16,17
However, most symptomatic malunions are complex three-dimensional
deformities, which can usually only be treated by prosthetic
replacement (Fig. 35-38). The integrity of the
glenoid articular surface determines whether humeral hemiarthroplasty
or total shoulder arthroplasty is performed. The chief indication is
for pain relief and the functional gains are often minimal. Extensive
capsular excision is usually required, and any associated rotator cuff
tears should be repaired.
tuberosity osteotomy can be avoided by using a small stem that is
shifted in the medullary canal to compensate for the tuberosity
malposition or by the use of an eccentric modular humeral head. If the
normal relationship between the tuberosities and the humeral head
cannot be achieved, either a tuberosity osteotomy and conventional
arthroplasty, or a reverse shoulder arthroplasty should be performed.
There may also be a role for the use of a resurfacing humeral
hemiarthroplasty in selected cases, as this does not require the use of
an intramedullary stem for fixation. Three quarters of the humeral head
must remain after reaming for this technique to be used, to allow
secure fixation and prosthetic bone ingrowth.
complex multipart malunions is scarce, but the results of prosthetic
arthroplasty are inferior to those of prosthetic treatment of similar
acute fractures. In particular, the requirement for tuberosity
osteotomy is associated with a poor prognosis.26 Pain relief is usually achieved, but shoulder range of motion and strength are often limited.*
The results of the use of reverse shoulder arthroplasty for the
treatment of severe malunion are still largely unknown in the longer
term, although this is a promising new technique and the results of
comparative outcome studies are awaited.30
multifactorial: Although capsular contracture is usually the main cause
of refractory stiffness, other factors may include fracture malunion,
complex regional pain syndrome, thoracic outlet syndrome, mechanical
impingement of implants, and rotator cuff dysfunction from impingement
or tears. These factors are poorly described in the contemporary
literature but may nevertheless be contributory to persistent stiffness
after fracture.
movement in a “capsular pattern,” with generalized stiffness but
selectively greater loss of shoulder abduction and external rotation.
The initial treatment is nonoperative with shoulder rehabilitation to
attempt to regain movement by selective stretching exercises. Most
patients improve to a degree on this regime, and recovery of movement
is often protracted over the first year after injury. A plateau in
recovery is usually heralded by the presence of a firm “woody” feel on
terminal stretching exercises, suggesting a mechanical block to
movement. Distension arthrography is useful in stretching and rupturing
the capsule in idiopathic adhesive
capsulitis, but it is the author’s experience that this procedure is less effective in the post-traumatic shoulder.
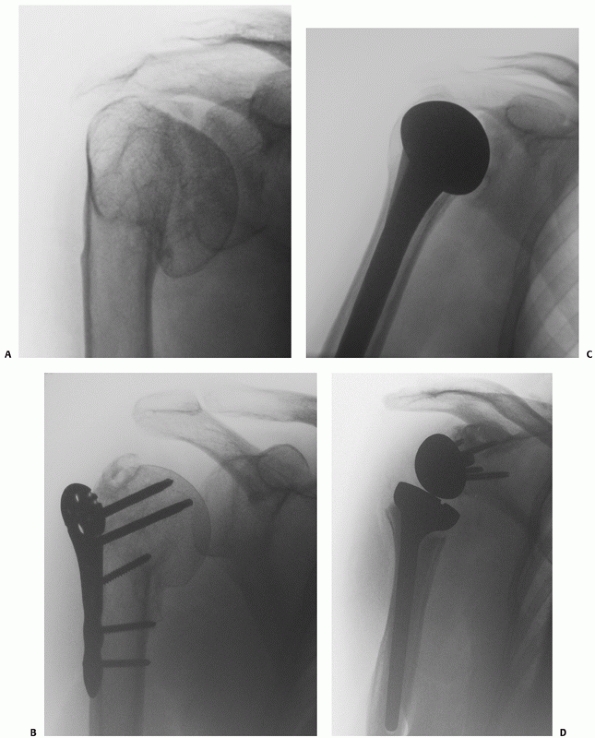 |
|
FIGURE 35-38 Symptomatic malunion may occur after nonoperative treatment (A) or poor operative reduction and fixation (B).
In most instances, treatment should involve humeral head replacement avoiding osteotomy of the greater tuberosity if possible (C). In this case, treatment was followed by symptomatic loosening at 3 years with massive rotator cuff tear. A revision to a reverse shoulder arthroplasty was successful in eliminating shoulder pain and improving function (D). |
whether the stiffness is because of soft tissue contracture or the
malunion itself. An examination under anesthesia under fluoroscopy
followed by an arthroscopic examination of the shoulder is often
required to distinguish these apart. If the malunion is considered to
be the cause of the stiffness, it is unlikely that a soft tissue
release will be effective and consideration is given to corrective
osteotomy as described previously.
without malunion, treatment with manipulation under anesthesia is
usually performed. This procedure is contraindicated in patients with
uncertain fracture healing and in patients with severe osteoporosis,
where there is a substantial risk of humeral shaft fracture during
manipulation. If manipulation is unsuccessful in regaining sufficient
movement, this should be followed by arthroscopic release of capsulitic
tissue from the rotator interval, circumferential intra-articular
capsular releases, subacromial decompression, and removal of impinging
metalwork.37,128,180
It is important to check for restoration of movement at each stage of
the release and to measure the final on-table range of movement at the
end of the procedure. The use of a continuous passive movement machine
with regional anesthesia may be useful in retaining movement in the
early postoperative period. Prolonged physiotherapy is often required
thereafter to consolidate the improved range of movement.
surgical repair using open methods. This is because of the rich
vascularity to this region and the good soft tissue coverage.50,136
The precise prevalence of this complication is difficult to evaluate,
because most reported case series of operative treatment are
retrospective. Although infection is usually regarded to be a
postsurgical complication, it may occasionally develop after
nonoperative treatment. This is more likely in thin, debilitated
patients, either from infection of the fracture hematoma, or in those
with displaced surgical neck fractures from pressure on the anterior
soft tissues.
|
TABLE 35-14 Currently Unanswered Questions in the Treatment of Proximal Humeral Fractures
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
risk is likely to be increased in thin and debilitated patients, with a
more severe soft tissue injury and grade of fracture, prolonged surgery
time, poor surgical technique, and operator inexperience. It is
important to distinguish superficial from deep infections. Superficial
infections are common, confined to the integumental layers, and do not
form a purulent collection. Superficial pin track infections are a
particularly common complication of percutaneous pinning of fractures.133,152
In contrast, deep infections often form a sinus with a deep purulent
collection that extends to the implant. These fail to resolve without
further surgical treatment. Ultrasound and MRI may be useful in
assessing for the presence of a deep collection.
growth of pathogenic organisms invariably resolve with antibiotic
therapy. It is often difficult to distinguish between a superficial
infection and a wound hematoma, especially if cultures are equivocal.
Broad-spectrum antibiotic therapy and topical dressings are frequently
given empirically following discharge from hospital, and most
superficial infections resolve on this regimen. More severe superficial
infections should be treated with parenteral antibiotics, guided by
wound cultures. An ultrasound-guided aspirate is useful in
distinguishing a deep purulent infection from a sterile wound hematoma.
Large sterile wound hematomas require surgical drainage, as wound
dehiscence may otherwise occur, with the risk of subsequent bacterial
colonization and deep infection.
as with any implant-related infection. Early sepsis with a stable
implant should be treated with a protocol of repeated surgical
irrigation and debridement and with prolonged parenteral and then oral
antibiotic therapy. The sepsis may be refractory to this treatment
protocol, and in these circumstances a radical débridement with implant
removal may be required to eradicate the infection, thereby allowing
later revision surgery.
humeral head arthroplasty. It may follow a transient bacteremia, and
the organism may be of low virulence or be antibiotic resistant.54,288
Débridement, metalwork removal, spacer insertion, and antibiotic
therapy may help to suppress or eradicate infection. Delayed
reimplantation may be possible if the infection can be eradicated.54,288
the aging population, which heal with a pain-free, functional shoulder
after nonoperative treatment. There are many unanswered questions in
the treatment of the minority of more complex injuries and those that
occur in younger and more active patients (Table 35-14).
To meet this challenge, the aim must be to produce more robust and
reliable standardized systems of classification and patient-orientated
outcome measures. Only when these are available will surgeons be able
to collaborate to produce the well-designed comparative clinical
outcome studies, to enable proper evidence-based management of these
injuries.
RB, Stansfield BW, Nunn T, et al. Reattachment of the tuberosities of
the humerus following hemiarthroplasty for four-part fracture. J Bone
Joint Surg Br 2006; 88B:1539-1544.
AO, Ikpeme JO. The results of internal fixation of three- and four-part
proximal humeral fractures with the Polarus nail. Injury
2001;32:115-121.
J, Schurmann M, Stahel P, et al. Analysis of efficacy and failure in
proximal humerus fractures treated with locking plates. J Orthop Trauma
2007;21:676-681.
SA, Sperling JW, Sanchez-Sotelo J, et al. Shoulder arthroplasty for
proximal humeral nonunions. J Shoulder Elbow Surg 2002;11:114-121.
M, Thet MM, Chambler AF. Arthroscopic treatment for malunion of a
head-splitting proximal humeral fracture: the need for adequate initial
radiologic investigation. J Shoulder Elbow Surg 2007;16:e1-e2.
ERH. Four-part proximal humeral fractures: ORIF. In: Warner JJP,
Iannotti JP, Gerber C, eds. Complex and Revision Problems in Shoulder
Surgery. 2nd Ed. Philadephia: Lippincott-Raven Publishers, 2005,
289-309.
C, Lingenfelter E, Fischer F, et al. Mechanism of injury and morphology
of the greater tuberosity fracture. J Shoulder Elbow Surg
2006;15:140-147.
SP, Andrisani D, Ramsey M, et al. The parachute technique: valgus
impaction osteotomy for two-part fractures of the surgical neck of the
humerus. J Bone Joint Surg Am 2001;83A(suppl 2, pt 1):38-42.
YM, Brincat M, Galea R, et al. The epidemiology of osteoporotic
fractures in a Mediterranean country. Calcif Tissue Int 1994;54:365-369.
JR, Burkhart SS. Arthroscopic capsular release after hemiarthroplasty
of the shoulder for fracture: a new treatment paradigm. Arthroscopy
2005;21:1150.
JD, Hertel R. Initial postfracture humeral head ischemia does not
predict development of necrosis. J Shoulder Elbow Surg 2008;17:2-8.
E, Zoppi FA, Ferreira Filho AA, et al. Surgical treatment of varus
malunion of the proximal humerus with valgus osteotomy. J Shoulder
Elbow Surg 2007;16: 55-59.
PK, Iannotti JP. Treatment of proximal humerus fracture malunion with
prosthetic arthroplasty. Instr Course Lect 1998;47:135-140.
PK, Iannotti JP, Norris TR, et al. Operative treatment of malunion of a
fracture of the proximal aspect of the humerus. J Bone Joint Surg Am
1998;80A: 1484-1497.
J, Charalambides C, Aderinto J, et al. Early failure of intramedullary
nailing for proximal humeral fractures. Injury 2000;31:789-792.
J, Adler LM, Blank JE, et al. Evaluation of the Neer system of
classification of proximal humeral fractures with computerized
tomographic scans and plain radiographs. J Bone Joint Surg Am
1996;78A:1371-1375.
ES, Lundh I, Ringqvist I. Physiotherapy after fracture of the proximal
end of the humerus. Comparison between two methods. Scand J Rehabil Med
1984;16:11-16.
DN, de Beer JF, van Rooyen KS. The bony partial articular surface
tendon avulsion lesion: an arthroscopic technique for fixation of the
partially avulsed greater tuberosity fracture. Arthroscopy 2007;23:786.
JM, Pajarinen J, Savolainen V. Internal fixation of proximal humeral
fractures with a locking compression plate: a retrospective evaluation
of 72 patients followed for a minimum of 1 year. Acta Orthop Scand
2004;75:741-745.
S, Dahlback LO. Nerve injuries in dislocations of the shoulder joint
and fracture of the neck of the humerus. Acta Chir Scand 136, 461-466.
1970.
P, Caligaris-Cordero B, Payeur F, et al. [Prognostic factors during
rehabilitation after shoulder prostheses for fracture]. Rev Chir Orthop
Reparatrice Appar Mot 1999; 85:106-116.
P, Chuinard C, Le Huec JC, et al. Proximal humerus fracture sequelae:
impact of a new radiographic classification on arthroplasty. Clin
Orthop Relat Res 2006;442: 121-130.
P, Krishnan SG, Tinsi L, et al. Tuberosity malposition and migration:
reasons for poor outcomes after hemiarthroplasty for displaced
fractures of the proximal humerus. J Shoulder Elbow Surg
2002;11:401-412.
P, Trojani C, Walch G, et al. Shoulder arthroplasty for the treatment
of the sequelae of fractures of the proximal humerus. J Shoulder Elbow
Surg 2001;10: 299-308.
P, Walch G. The three-dimensional geometry of the proximal humerus.
Implications for surgical technique and prosthetic design. J Bone Joint
Surg Br 1997;79: 857-865.
P, Watkinson D, Hatzidakis AM, et al. Neer Award 2005: The Grammont
reverse shoulder prosthesis: results in cuff tear arthritis, fracture
sequelae, and revision arthroplasty. J Shoulder Elbow Surg
2006;15:527-540.
CM, Renard R, Levine RG, et al. Effect of displacement of fractures of
the greater tuberosity on the mechanics of the shoulder. J Bone Joint
Surg Br 2001;83B: 1056-1062.
U, Fremerey RW, Skutek M, et al. [Hemi-arthroplasty—primary or
secondary measure for 3- and 4-fragment fractures of the proximal
humerus in the elderly?]. Unfallchirurg 1996;99:656-664.
U, Skutek M, Fremerey RW, et al. Outcome after primary and secondary
hemiarthroplasty in elderly patients with fractures of the proximal
humerus. J Shoulder Elbow Surg 1998;7:479-484.
CH, Revell WJ, Heatley FW. Vascularity of the humeral head after
proximal humeral fractures. An anatomical cadaver study. J Bone Joint
Surg Br 1993;75B: 132-136.
SS. Arthroscopic subscapularis tenolysis: a technique for treating
refractory glenohumeral stiffness following open reduction and internal
fixation of a displaced three-part proximal humerus fracture.
Arthroscopy 1996;12:87-91.
WZ Jr, Skedros JG, O’Rourke PJ, et al. A novel double-row rotator cuff
repair exceeds strengths of conventional repairs. Clin Orthop Relat Res
2007;461:106-113.
ER, Ahmad CS. Arthroscopic reduction and suture anchor fixation for a
displaced greater tuberosity fracture: a case report. J Shoulder Elbow
Surg 2007;16:e6-e9.
E, de Miquel I, de la Cruz JJ, et al. Percutaneous fixation of
displaced proximal humeral fractures: indications based on the
correlation between clinical and radiographic results. J Shoulder Elbow
Surg 2007;16:774-781.
R, Catagni MA, Guerreschi F. Applications of the Ilizarov method in the
humerus. Lengthenings and nonunions. Hand Clin 1993;9:729-739.
O, Uslu M, Acar HI, et al. Is there a safe area for the axillary nerve
in the deltoid muscle? A cadaveric study. J Bone Joint Surg Am
2006;88A:2395-2399.
CY, Chao EK, Tu YK, et al. Closed management and percutaneous fixation
of unstable proximal humerus fractures. J Trauma 1998;45:1039-1045.
EA. The Shoulder. Rupture of the Supraspinatus Tendon and Other Lesions
in or about the Subacromial Bursa. Boston: privately printed, 1934.
EA. The Shoulder. Rupture of the Supraspinatus Tendon and Other Lesions
in or about the Subacromial Bursa. Boston: Thomas Todd, 2008.
S, Levy O, Brownlaw HC. Resurfacing arthroplasty of the shoulder.
Techniques in shoulder and elbow surgery 2003;4:199-210.
CN. Tension-band wiring supplemented by lag-screw fixation of proximal
humerus fractures: a modified technique. Orthop Rev 1994;(suppl):19-23.
CN, Levine D, Pagnani MJ. Internal fixation of proximal humerus
fractures using the screw-tension band technique. J Orthop Trauma
1994;8:23-27.
JS, Reig S, Trojani C, et al. The management of infection in
arthroplasty of the shoulder. J Bone Joint Surg Br 2004;86B:65-69.
CM, Cattermole H, McQueen MM. Impacted valgus fractures B1.1. of the
proximal humerus. The results of nonoperative treatment. J Bone Joint
Surg Br 2002; 84B:504-508.
CM, Garg A, McQueen MM. The translated two-part fracture of the
proximal humerus. Epidemiology and outcome in the older patient. J Bone
Joint Surg Br 2001;83:799-804.
CM, McQueen MM. The impacted varus A2.2. proximal humeral fracture:
prediction of outcome and results of nonoperative treatment in 99
patients. Acta Orthop Scand 2004;75:736-740.
CM, McQueen MM. Nonunions of the proximal humerus: their prevalence and
functional outcome. J Trauma 2008;64:1517-1521.
C, Darbelley L, Touchard O, et al. [Proximal 4-part humerus fractures
treated by antegrade nailing with self-stabilizing screws: 31 cases].
Rev Chir Orthop Reparatrice Appar Mot 2003;89:507-514.
Laat EA, Visser CP, Coene LN, et al. Nerve lesions in primary shoulder
dislocations and humeral neck fractures. A prospective clinical and EMG
study. J Bone Joint Surg Br 1994;76B:381-383.
MJ, Brems JJ, Williams GR Jr, et al. Evaluation and management of
valgus impacted four-part proximal humerus fractures. Clin Orthop Relat
Res 2006;442: 109-114.
P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of
proximal humeral fractures. J Bone Joint Surg Am 2007;89A:1700-1709.
DM, et al. Posttraumatic changes of the proximal humerus: malunion,
nonunion, and osteonecrosis: treatment with modular hemiarthroplasty or
total shoulder arthroplasty. J Shoulder Elbow Surg 1993;2:11-21.
AM, Faber J, Lynnerup N, et al. The effect of calcium and vitamin D3
supplementation on the healing of the proximal humerus fracture: a
randomized placebocontrolled study. Calcif Tissue Int 2004;75:183-188.
L, Grimberg J, Cazeau C, et al. A new internal fixation technique for
fractures of the proximal humerus—the Bilboquet device: a report on 26
cases. J Shoulder Elbow Surg 2000;9:279-288.
G, Kelly I, Vigder F, et al. A three-dimensional classification for
fractures of the proximal humerus. J Bone Joint Surg Br 2004;86:413-425.
G, Safuri H, Salami J, et al. Natural history of complex fractures of
the proximal humerus using a three-dimensional classification system. J
Shoulder Elbow Surg 2008.
KA, Ong CC, Walsh M, et al. Early complications in proximal humerus
fractures OTA types 11. Treated with locked plates. J Orthop Trauma
2008;22:159-164.
F, Boldin C, Schippinger G, et al. A new locking plate for unstable
fractures of the proximal humerus. Clin Orthop Relat Res 2005;176-181.
RD, Hedley AK, Eckardt JJ. Anterior fracture-dislocations of the
shoulder: pitfalls in treatment. J Trauma 1984;24:363-367.
T, Stromsoe K, Blucher J, et al. Fractures in the proximal humerus:
functional outcome and evaluation of 70 patients treated in hospital.
Arch Orthop Trauma Surg 2005;125:310-316.
E. Fractures of the proximal humerus. In: Bucholz RW, Heckman JD, eds.
Rockwood and Green’s Fractures in Adults. 5th Ed. Philadelphia:
Lippincott, Williams & Wilkins, 2001, 997-1040.
EL, Bigliani LU, April EW. An anatomic study of the musculocutaneous
nerve and its relationship to the coracoid process. Clin Orthop Relat
Res 1989;166-171.
EL, Cuomo F, Maday MG, et al. Open reduction and internal fixation of
two-part displaced fractures of the greater tuberosity of the proximal
part of the humerus. J Bone Joint Surg Am 1991;73A:1213-1218.
MA, Greenwald DP, Markee BA, et al. Biomechanical effects of
malposition of tuberosity fragments on the humeral prosthetic
reconstruction for four-part proximal humerus fractures. J Shoulder
Elbow Surg 2001;10:321-326.
MA, Mighell MA. Techniques and principles of tuberosity fixation for
proximal humeral fractures treated with hemiarthroplasty. J Shoulder
Elbow Surg 2004;13: 239-247.
MA, Ondrovic LE, Markee BA, et al. Stability of tuberosity reattachment
in proximal humeral hemiarthroplasty. J Shoulder Elbow Surg
2002;11:413-420.
LH, Sojbjerg JO, Sneppen O. Shoulder arthroplasty in complex acute and
chronic proximal humeral fractures. Orthopedics 1991;14:949-954.
C, McQueen MM, CourtBrown CM. Minimally displaced proximal humeral
fractures: epidemiology and outcome in 507 cases. Acta Orthop Scand
2003;74: 580-585.
LM, Williams GR Jr, Fenlin JM Jr, et al. Outcome of open reduction and
internal fixation of surgical neck nonunions of the humerus. J Orthop
Trauma 2004;18:63-67.
RA, Sciulli R, Daffner RH, et al. Defining the relationship between
rotator cuff injury and proximal humerus fractures. Clin Orthop Relat
Res 2007;458:70-77.
RA, Zeiders GJ, Altman GT. Two-incision technique for treatment of
complex proximal humerus fractures. J Orthop Trauma 2005;19:734-740.
MJ, Boraiah S, Helfet DL, et al. Indirect medial reduction and strut
support of proximal humerus fractures using an endosteal implant. J
Orthop Trauma 2008;22: 195-200.
MJ, Griffith MH, Dines JS, et al. The extended anterolateral acromial
approach allows minimally invasive access to the proximal humerus. Clin
Orthop Relat Res 2005; 123-129.
MJ, Griffith MH, Dines JS, et al. A minimally invasive approach for
plate fixation of the proximal humerus. Bull Hosp Jt Dis
2004;621-2.:18-23.
MJ, Lorich DG, Werner CM, et al. Second-generation concepts for locked
plating of proximal humerus fractures. Am J Orthop 2007;36:460-465.
MJ, Voos JE, Wanich T, et al. Vascular implications of minimally
invasive plating of proximal humerus fractures. J Orthop Trauma
2006;20:602-607.
MJ, Weil Y, Barker JU, et al. The importance of medial support in
locked plating of proximal humerus fractures. J Orthop Trauma
2007;21:185-191.
GM, Taverna E. Arthroscopic treatment of rotator cuff tear and greater
tuberosity fracture nonunion. Arthroscopy 1996;12:242-244.
GM, Taverna E, Hammerman SM. Arthroscopic treatment of acute traumatic
anterior glenohumeral dislocation and greater tuberosity fracture.
Arthroscopy 1999; 15:648-650.
A, Warner JP. Hemiarthroplasty for Management of Complex Proximal
Humerus Fractures: Preoperative Planning and Surgical Solutions. In:
Warner JJP, Flatow EL, Iannotti JP, eds. Complex and revision problems
in shoulder surgery. Philadelphia: Lippincott-Raven, 2005, pp. 2005.
C, Hersche O, Berberat C. The clinical relevance of posttraumatic
avascular necrosis of the humeral head. J Shoulder Elbow Surg
1998;7:586-590.
C, Lambert SM. Allograft reconstruction of segmental defects of the
humeral head for the treatment of chronic locked posterior dislocation
of the shoulder. J Bone Joint Surg Am 1996;78A:376-382.
C, Lambert SM, Hoogewoud HM. Absence of avascular necrosis of the
humeral head after posttraumatic rupture of the anterior and posterior
humeral circumflex arteries. A case report. J Bone Joint Surg Am
1996;78A:1256-1259.
C, Schneeberger AG, Vinh TS. The arterial vascularization of the
humeral head. An anatomical study. J Bone Joint Surg Am
1990;72A:1486-1494.
C, Terrier F, Ganz R. The role of the coracoid process in the chronic
impingement syndrome. J Bone Joint Surg Br 1985;67B:703-708.
C, Werner CM, Vienne P. Internal fixation of complex fractures of the
proximal humerus. J Bone Joint Surg Br 2004;86B:848-855.
GI, Rockwood CA Jr. The terrible triad: anterior dislocation of the
shoulder associated with rupture of the rotator cuff and injury to the
brachial plexus. J Shoulder Elbow Surg 1995;41:51-53.
H, Yamamoto K, Ohshiro H, et al. Changing incidence of hip, distal
radius, and proximal humerus fractures in Tottori Prefecture, Japan.
Bone 1999;24:265-270.
SC, Chapman JA, Choudhury G, et al. Retrograde fixation of fractures of
the neck and shaft of the humerus with the “Halder humeral nail.”
Injury 2001;32: 695-703.
HH, Gibson JN, Madhok R. Interventions for treating proximal humeral
fractures in adults. Cochrane Database Syst Rev 2003;CD000434.
KH. Blood supply of intraarticular fractures of the humeral head: an
anatomical and biomechanical study. In: Proceedings of the 14th
Congress of the European Society for Shoulder and Elbow Surgery,
Lisbon, 2000.
RJ, Angelo RL. Displaced proximal humeral fractures. Selecting
treatment, avoiding pitfalls. Orthop Clin North Am 1987;18:421-431.
RJ, Bell RH, Gurr K. The three-part fracture of the proximal part of
the humerus. Operative treatment. J Bone Joint Surg Am
1986;68A:1410-1414.
WL, Jupiter JB, Kristiansen TK, et al. Nonunion of the proximal
humerus. A review of 25 cases. J Orthop Trauma 1990;4:424-431.
R, Kampshoff J, Kinner B, et al. [Treatment of dislocated 3- and 4-part
fractures of the proximal humerus with an angle-stabilizing fixation
plate]. Unfallchirurg 2004; 107:769-782.
O, Gerber C. Iatrogenic displacement of fracture-dislocations of the
shoulder. A report of seven cases. J Bone Joint Surg Br 1994;76B:30-33.
D Jr, Saunders DT, Johnson MP, et al. Percutaneous fixation of proximal
humeral fractures. Clin Orthop Relat Res 2000;97-104.
R, Hempfing A, Stiehler M, et al. Predictors of humeral head ischemia
after intracapsular fracture of the proximal humerus. J Shoulder Elbow
Surg 2004;13: 427-433.
R, Knothe U, Ballmer FT. Geometry of the proximal humerus and
implications for prosthetic design. J Shoulder Elbow Surg
2002;11:331-338.
M, Baumgaertel F, Gehling H, et al. Plate fixation of proximal humeral
fractures with indirect reduction: surgical technique and results
utilizing three shoulder scores. Injury 1999;30:453-462.
SA, Mawson SJ, Stanley D. Rehabilitation after two-part fractures of
the neck of the humerus. J Bone Joint Surg Br 2003;85B:419-422.
GB, Schenk T, Williams GR, et al. Arthroscopic capsular release for the
treatment of refractory postoperative or post-fracture shoulder
stiffness. J Bone Joint Surg Am 2001;83A:1682-1687.
E, Gagey O, Mestdagh H, et al. The blood supply of the deltoid muscle.
Application to the deltoid flap technique. Surg Radiol Anat
1998;20:161-165.
JP, Gabriel JP, Schneck SL, et al. The normal glenohumeral
relationships. An anatomical study of 140 shoulders. J Bone Joint Surg
Am 1992;74A:491-500.
GA, Sysenko I, Shved SI, et al. [Transosseous osteosynthesis in
treating fracture-dislocations of the shoulder]. Ortop Travmatol Protez
1982;46-48.
H, Warner JJ, Jakob RP. Percutaneous stabilization of unstable
fractures of the humerus. J Bone Joint Surg Am 1992;74A:508-515.
RP, Miniaci A, Anson PS, et al. Four-part valgus impacted fractures of
the proximal humerus. J Bone Joint Surg Br 1991;73B:295-298.
J, Greig M, Peuker ET, et al. The posterior subdeltoid approach: a
modified access to the posterior glenohumeral joint. J Shoulder Elbow
Surg 2001;10:265-268.
AG, Albrechtsen J. The use of computed tomography with two- and
three-dimensional reconstructions in the diagnosis of three- and
four-part fractures of the proximal humerus. Clin Radiol
1994;49:800-804.
S, Ankem H, Sanghavi S. Anatomical considerations for percutaneous
proximal humeral fracture fixation. Injury 2004;35:1133-1136.
P, Palvanen M, Niemi S, et al. Osteoporotic fractures of the proximal
humerus in elderly Finnish persons: sharp increase in 1970 to 1998 and
alarming projections for the new millennium. Acta Orthop Scand
2000;71:465-470.
V, Alekberov C, Baran O, et al. Open fractures of the proximal humerus
treated with the Ilizarov method: 12 patients followed 3 to 8 years.
Acta Orthop Scand 2002;73:460-464.
JD, Parsons BO, Flatow EL, et al. Outcomes after percutaneous reduction
and fixation of proximal humeral fractures. J Shoulder Elbow Surg
2007;16:330-338.
JL, Browner WS, Seeley DG, et al. Risk factors for fractures of the
distal forearm and proximal humerus. The Study of Osteoporotic
Fractures Research Group. Am J Epidemiol 1992;135:477-489.
CB, Tanner SL, Tolan SJ. SLAP tear associated with a minimally
displaced proximal humerus fracture. Arthroscopy 2007;23:1362-1363.
S, Bolukbasi S, Bayar A, et al. Proximal humeral fractures with minimal
displacement treated conservatively. Int Orthop 2004;28:231-234.
M, Biberthaler P, Braunstein V, et al. [Treatment of proximal humeral
fractures with the PHILOS angular stable plate. Presentation of 225
cases of dislocated fractures]. Unfallchirurg 2006;109:1032-1040.
LA, Robinson CM, Will E, Whittaker R. Assessment of axillary nerve
function and functional outcome after fixation of complex proximal
humerus fractures using the extended deltoid-splitting approach. Injury
2008.
DH, Clavert P, Warner JJ. Displaced periprosthetic humeral fracture
treated with functional bracing: a report of two cases. J Shoulder
Elbow Surg 2005;14:221-223.
KC, Rhee KJ, Shin HD, et al. Arthroscopic fixation for displaced
greater tuberosity fracture using the suture-bridge technique.
Arthroscopy 2008;24:120-123.
SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with
minimally displaced greater tuberosity fracture. Arthroscopy
2000;16:695-700.
RA, Mayne JA. Comminuted fractures and fracture-dislocations involving
the articular surface of the humeral head. J Bone Joint Surg Am
1957;39A:1343-1355.
A, Wallace WA. Closed percutaneous K-wire stabilization for displaced
fractures of the surgical neck of the humerus. Injury 1990;21:209-212.
GM, Steriopoulos K, Damilakis J, et al. The position of the axillary
nerve in the deltoid muscle. A cadaveric study. Acta Orthop Scand
1999;70:9-11.
A, Apostolou CD, Taneja T, et al. Fixation of proximal humerus
fractures using the PHILOS plate: early experience. Clin Orthop Relat
Res 2006;442:115-120.
K, Sanders R, Zuckerman J, et al. Modified tension band wiring of
displaced surgical neck fractures of the humerus. J Shoulder Elbow Surg
2, 85-92. 1993.
KJ, Gallagher MA, Marsicano JG, et al. Functional outcome after
minimally displaced fractures of the proximal part of the humerus. J
Bone Joint Surg Am 1997;79A: 203-207.
F, Irenberger A, Lechner C, et al. [Comparison of open versus
percutaneous treatment for humeral head fracture]. Unfallchirurg
2006;109:406-410.
F, Schwaiger R, Wambacher M, et al. Outcome after primary
hemiarthroplasty for fracture of the head of the humerus. A
retrospective multicenter study of 167 patients. J Bone Joint Surg Br
2004;86B:217-219.
FG, Huebschle L, Hertel R. Reattachment of the tuberosities with cable
wires and bone graft in hemiarthroplasties done for proximal humeral
fractures with cable wire and bone graft: 58 patients with a 22-month
minimum follow-up. J Orthop Trauma 2007;21:682-686.
B, Kofoed H. Transcutaneous reduction and external fixation of
displaced fractures of the proximal humerus. J Bone Joint Surg
1988;70B:821-824.
B. External fixation of proximal humerus fracture. Clinical and cadaver
study of pinning technique. Acta Orthop Scand 1987;58:645-648.
B. Treatment of displaced fractures of the proximal humerus:
transcutaneous reduction and Hoffmann’s external fixation. Injury
1989;20:195-199.
B, Angermann P, Larsen TK. Functional results following fractures of
the proximal humerus. A controlled clinical study comparing two periods
of immobilization. Arch Orthop Trauma Surg 1989;108:339-341.
B, Kofoed H. External fixation of displaced fractures of the proximal
humerus. Technique and preliminary results. J Bone Joint Surg Br
1987;69B:643-646.
M, Brostrom LA, Soderlund V. Retroversion of the humeral head in the
normal shoulder and its relationship to the normal range of motion.
Clin Orthop Relat Res 1990;113-117.
BK, Goertzen DJ, O’Brien PJ, et al. Biomechanical evaluation of
proximal humeral fracture fixation supplemented with calcium phosphate
cement. J Bone Joint Surg Am 2002;84A:951-961.
GY, Rouleau DM, Berry GK, et al. Percutaneous humeral plating of
fractures of the proximal humerus: results of a prospective multicenter
clinical trial. J Orthop Trauma 2008;22:153-158.
L, Brozska R, Toussaint B, et al. The outcome and structural integrity
of arthroscopic rotator cuff repair with use of the double-row suture
anchor technique. J Bone Joint Surg Am 2007;89A:1533-1541.
B, MacDermid J, Drosdowech D, Faber KJ. Proximal humeral fractures: a
systematic review of treatment modalities. J Shoulder Elbow Surg
2008;17:42-54.
SH, Dargent-Molina P, Breart G. Risk factors for fractures of the
proximal humerus: results from the EPIDOS prospective study. J Bone
Miner Res 2002;17:817-825.
MM, Babinet A, Fayad F, et al. Immediate mobilization compared with
conventional immobilization for the impacted nonoperatively treated
proximal humeral fracture. A randomized controlled trial. J Bone Joint
Surg Am 2007;89A:2582-2590.
A, Cassar-Pullicino VN. Avulsion of the lesser tuberosity with
intra-articular injury of the glenohumeral joint. Injury
1996;27:742-745.
B, Pereira D, Rosen J. Avulsion fractures of the lesser tuberosity of
the humerus in adolescents: review of the literature and case report. J
Orthop Trauma 2005;19: 349-352.
O, Copeland SA. Cementless surface replacement arthroplasty of the
shoulder: 5- to 10-year results with the Copeland mark-2 prosthesis. J
Bone Joint Surg Br 2001; 83B:213-221.
O, Pritsch M, Oran A, et al. A wide and versatile combined surgical
approach to the shoulder. J Shoulder Elbow Surg 1999;8:658-659.
H, Bewer A, Korner J, et al. [Conservative treatment of dislocated
proximal humeral fractures]. Zentralbl Chir 2001;126:205-210.
H, Hepp P, Korner J, et al. Proximal humeral fractures: how stiff
should an implant be? A comparative mechanical study with new implants
in human specimens. Arch Orthop Trauma Surg 2003;1232-3.:74-81.
H, Hepp P, Rose T, et al. [The angle stable
locking-proximal-humerus-plate LPHP. for proximal humeral fractures
using a small anterior-lateral-deltoid-splitting-approach: technique
and first results]. Zentralbl Chir 2004;129:43-48.
PJ, Mugglestone A, Whitton J. Electrotherapy and the management of
minimally displaced fracture of the neck of the humerus. Injury
1992;23:323-327.
M, Heitkemper S, Parsch D, et al. Influence of the design of the
prosthesis on the outcome after hemiarthroplasty of the shoulder in
displaced fractures of the head of the humerus. J Bone Joint Surg Br
2006;88B:345-350.
CC, Chang MW, Lin GT. Intramedullary pinning with tension-band wiring
for surgical neck fractures of the proximal humerus in elderly
patients. Kaohsiung J Med Sci 2004;20:538-545.
P, Guity MR, Bellumore Y, et al. Shoulder arthroplasty for late
sequelae of proximal humeral fractures. J Shoulder Elbow Surg
2004;13:305-312.
JL, Slongo TF, Agel J, et al. Fracture and dislocation classification
compendium —2007: Orthopaedic Trauma Association classification,
database, and outcomes committee. J Orthop Trauma
2007;2110(suppl):S1-133.
C, Alt V, Hassanin H, et al. The arteries of the humeral head and their
relevance in fracture treatment. Surg Radiol Anat 2005;27:232-237.
DC, Espinosa N, Hertel R. Combined fracture of the greater and lesser
tuberosities with intact connection of the humeral head to the shaft. J
Trauma 2006;61: 206-208.
A, Kapur R, Maffulli N. Complex proximal humeral fractures in adults: a
systematic review of management. Injury 2001;32:363-372.
TW, Stedtfeld HW, Ewert A, et al. Stabilization of proximal humeral
fractures with an angular and sliding stable antegrade locking nail
Targon PH. J Bone Joint Surg Am 2003;85A(suppl 4):136-146.
T, Sugaya H, Uomizu M, et al. Humeral insertion of the supraspinatus
and infraspinatus. New anatomical findings regarding the footprint of
the rotator cuff. J Bone Joint Surg Am 2008;90A:962-969.
S, Chadha N, Sangwan S, et al. Open reduction and internal fixation of
proximal humeral fractures and fracture dislocations. J Bone Joint Surg
Br 1990;72B:1050-1052.
MA, Maar DC, Urquhart MW, et al. Avascular necrosis of the humeral head
treated by core decompression. A retrospective review. J Bone Joint
Surg Br 1993;75B: 785-788.
P, Ashwood N, Hamlet M. Early results for treatment of three-and
four-part fractures of the proximal humerus using the PHILOS plate
system. J Bone Joint Surg Br 2007;89B:1206-1209.
DA, Herbert KJ, Morris AM, et al. The deltoid muscle flap: anatomical
studies and case reports. Br J Plast Surg 1996;49:310-314.
NK, Schickendantz MS, Regan WD, et al. Operative treatment of nonunion
of surgical neck fractures of the humerus. Clin Orthop Relat Res
1995;200-205.
CS. Four-segment classification of proximal humeral fractures: purpose
and reliable use. J Shoulder Elbow Surg 2002;11:389-400.
CS. Displaced proximal humeral fractures. II. Treatment of three-part
and four-part displacement. J Bone Joint Surg Am 1970;52A:1090-1103.
C. Four-segment classification of displaced proximal humeral fractures.
Am Acad Orthop Surg Instr Course Lect 1975;chapter 9:160-168.
K, Muhr G, Breitfuss H. [Primary humerus head replacement in dislocated
proximal humeral fracture. Indications, technique, results]. Orthopade
1992;21: 140-147.
TV, Center JR, Sambrook PN, et al. Risk factors for proximal humerus,
forearm, and wrist fractures in elderly men and women: the Dubbo
Osteoporosis Epidemiology Study. Am J Epidemiol 2001;153:587-595.
A, Petersson CJ. Shoulder injuries common in alcoholics. An analysis of
413 injuries. Acta Orthop Scand 1996;67:364-366.
TR, Green A, McGuigan FX. Late prosthetic shoulder arthroplasty for
displaced proximal humerus fractures. J Shoulder Elbow Surg
1995;4:271-280.
L, Walch G, Levigne CH, et al. Fracture of the lesser tuberosity of the
humerus in adults. About 17 cases. J Shoulder Elbow Surg 1995;4:S30-S33.
TM, McKenna JV, Kenny P, et al. Concomitant injuries to the ipsilateral
shoulder in patients with a fracture of the diaphysis of the humerus. J
Bone Joint Surg Br 2008;90B:61-65.
PE, Ilchmann T. [Tension band osteosynthesis with absorbable cords in
proximal comminuted fractures of the humerus]. Unfallchirurg
1991;94:508-510.
N, Aoki M, Okamura K, et al. Ender nailing for unstable surgical neck
fractures of the humerus in elderly patients. Clin Orthop Relat Res
1996;330:173-180.
C, Petersson CJ. Clinical importance of comorbidity in patients with a
proximal humerus fracture. Clin Orthop Relat Res 2006;442:93-99.
C, Nordqvist A, Petersson CJ. Increased fragility in patients with
fracture of the proximal humerus: a case control study. Bone
2004;34:1072-1077.
C, Nordquist A, Petersson CJ. Long-term outcome of a proximal humerus
fracture predicted after 1 year: a 13-year prospective population-based
follow-up study of 47 patients. Acta Orthop 2005;76:397-402.
KC, Gorczyca JT. Displacement/screw cutout after open reduction and
locked plate fixation of humeral fractures. J Bone Joint Surg Am
2008;90A:233-240.
P, Bjorkenheim JM, Slatis P, et al. Operative treatment of severe
proximal humeral fractures. Acta Orthop Scand 1983;54:374-379.
M, Kannus P, Niemi S, et al. Update in the epidemiology of proximal
humeral fractures. Clin Orthop Relat Res 2006;442:87-92.
M, Kannus P, Parkkari J, et al. The injury mechanisms of osteoporotic
upper extremity fractures among older adults: a controlled study of 287
consecutive patients and their 108 controls. Osteoporos Int
2000;11:822-831.
AM, Dimakopoulos P, Tyllianakis M, et al. Valgus impacted proximal
humeral fractures and their blood supply after transosseous suturing.
Int Orthop 2004; 28:333-337.
RM, Mack LA, Wang KY, et al. Nondisplaced fractures of the greater
tuberosity of the humerus: sonographic detection. Radiology
1992;182:201-204.
P, Kutscha-Lissberg F, Lehr S, et al. The influence of displacement on
shoulder function in patients with minimally displaced fractures of the
greater tuberosity. Injury 2005;36:1185-1189.
D, Kwon YW, Zuckerman JD. Complications of humeral head replacement for
proximal humeral fractures. Instr Course Lect 2005;54:371-380.
R, Hoffmeyer P, Bednarkiewicz M, et al. Blunt injury to the subclavian
or axillary artery. J Am Coll Surg 1994;179:295-298.
C, Ray PS, Bhamra MS. Fixation of proximal humeral fractures with the
Polarus nail. J Shoulder Elbow Surg 2001;10:7-10.
S, Hvass I, Dalsgaard J, et al. Displaced proximal humeral fractures:
results of conservative treatment. Injury 1992;23:41-43.
WR, Hatem SF. Fractures of the greater tuberosity presenting as rotator
cuff abnormality: magnetic resonance demonstration. J Trauma
1998;44:670-675.
H, Hubner C, Schwaiger R. Minimally invasive reduction and
osteosynthesis of articular fractures of the humeral head. Injury
2001;32(suppl 1):SA25-SA32.
H, Povacz P, Frohlich R, et al. Percutaneous fixation of three-and
four-part fractures of the proximal humerus. J Bone Joint Surg Br
1997;79B:295-300.
H, Hubner C. Percutaneous treatment of proximal humerus fractures. In:
Levine WN, et al., eds. Fractures of the Shoulder Girdle. New York:
Marcel Decker, 2003.
H, Aschaver E, Poracz P, et al. Closed reduction and fixation of
articular fractures of the humeral head. Tech Shoulder Elbow Surg
2000;3:154-158.
S, Dahlstrom M, Dalen N. Water exercise versus instruction for
self-training following a shoulder fracture. Int J Rehabil Res
1992;15:327-333.
DD, Yuan J, Bigliani LU, et al. Three-dimensional analysis of the
proximal part of the humerus: relevance to arthroplasty. J Bone Joint
Surg Am 2000;82A: 1594-1602.
CM, Akhtar A, Mitchell M, et al. Complex posterior fracture-dislocation
of the shoulder. Epidemiology, injury patterns, and results of
operative treatment. J Bone Joint Surg Am 2007;89A:1454-1466.
CM, Bell KM, CourtBrown CM, et al. Locked nailing of humeral shaft
fractures. Experience in Edinburgh over a 2-year period. J Bone Joint
Surg Br 1992;74B: 558-562.
CM, Christie J. The 2-part proximal humeral fracture: a review of
operative treatment using two techniques. Injury 1993;24:123-125.
CM, Howes J, Murdoch H, et al. Functional outcome and risk of recurrent
instability after primary traumatic anterior shoulder dislocation in
young patients. J Bone Joint Surg Am 2006;88A:2326-2336.
CM, Kelly M, Wakefield AE. Redislocation of the shoulder during the
first 6 weeks after a primary anterior dislocation: risk factors and
results of treatment. J Bone Joint Surg Am 2002;84A:1552-1559.
CM, Khan L, Akhtar A, et al. The extended deltoid-splitting approach to
the proximal humerus. J Orthop Trauma 2007;21:657-662.
CM, Khan LA, Akhtar MA. Treatment of anterior fracture-dislocations of
the proximal humerus by open reduction and internal fixation. J Bone
Joint Surg Br 2006; 88B:502-508.
CM, Page RS. Severely impacted valgus proximal humeral fractures.
Results of operative treatment. J Bone Joint Surg Am 2003;85A:1647-1655.
CM, Page RS, Hill RM, et al. Primary hemiarthroplasty for treatment of
proximal humeral fractures. J Bone Joint Surg Am 2003;85A:1215-1223.
CM, Royds M, Abraham A, et al. Refractures in patients at least 45
years old. A prospective analysis of 22,060 patients. J Bone Joint Surg
Am 2002;84A:1528-1533.
CM, Teoh KH, Baker A, et al. Fractures of the lesser tuberosity of the
humerus in the adult: epidemiology and functional outome after
operative treatment. J Bone Joint Surg Am 2008.
PJ, Cockshott WP. Pseudarthrosis following proximal humeral fractures:
a possible mechanism. Skeletal Radiol 1986;15:21-24.
PS, Adams CR, Torchia ME, et al. Locking plate fixation for proximal
humeral fractures: initial results with a new implant. J Shoulder Elbow
Surg 2007;16:202-207.
DJ, McGrory JE. Percutaneous pinning of the proximal part of the
humerus. An anatomic study. J Bone Joint Surg Am 2001;83A:1695-1699.
R, Visconti V, Lombardi LV, et al. The block-bridge system: a new
concept and surgical technique to reconstruct articular surfaces and
tuberosities in complex proximal humeral fractures. J Shoulder Elbow
Surg 2008;17:29-36.
MB, Hay JG, Goel VK, et al. Active responses decrease impact forces at
the hip and shoulder in falls to the side. J Biomech 1999;32:993-998.
S, Natatsuchi Y, Latta L, et al. Distribution of bone mineral density
and bone strength of the proximal humerus. J Shoulder Elbow Surg
1994;3:234-242.
PI, Pedowitz RA, Mallon WJ, et al. Reliability and reproducibility of
radiographic interpretation of proximal humeral fracture pathoanatomy.
J Shoulder Elbow Surg 1997;6:60-69.
KM, Seeman E, Ugoni AM, et al. Age- and gender-specific rate of
fractures in Australia: a population-based study. Osteoporos Int
1999;10:240-247.
PA, Hintermann B, Koris MJ. Preoperative arthroscopic assessment of
fractures about the shoulder. Arthroscopy 1999;15:827-835.
M, Martinek V, Imhoff AB. Arthroscopic reconstruction of an isolated
avulsion fracture of the lesser tuberosity. Arthroscopy 2005;21:487-494.
AH. Proximal humeral fractures: open reduction internal fixation. In:
Wiss DA, ed. Master Techniques in Orthopaedic Surgery. Fractures. 2nd
Ed. Philadelphia: Lippincott-Raven, 2006, 37-49.
E, Karatosun V, Balci C, et al. Two-prong splint in the treatment of
proximal humeral fracture. Arch Orthop Trauma Surg 1999;119:368-370.
NL, Robinson CM. Mortality after low-energy fractures in patients aged
at least 45 years old. J Orthop Trauma 2005;19:396-400.
SI, Sysenko I. [Treatment of fractures of the proximal end of the
humerus in middle-aged and elderly patients by the Ilizarov method].
Vestn Khir Im I I Grek 1984; 132:80-82.
ML, Zuckerman JD, Lyon T, et al. The Neer classification system for
proximal humeral fractures. An assessment of interobserver reliability
and intraobserver reproducibility. J Bone Joint Surg Am
1993;75A:1745-1750.
KA, Gerber C. The reproducibility of classification of fractures of the
proximal end of the humerus. J Bone Joint Surg Am 1993;75A:1751-1755.
NS, Schwappach JR, Toby EB. Fracture-dislocation of the humerus with
intrathoracic displacement of the humeral head. A case report. J Bone
Joint Surg Am 1998; 80A:889-891.
F, Favard L, Oudet D, et al. Grammont inverted total shoulder
arthroplasty in the treatment of glenohumeral osteoarthritis with
massive rupture of the cuff. Results of a multicenter study of 80
shoulders. J Bone Joint Surg Br 2004;86B:388-395.
J, Berry G, Laflamme Y, et al. Percutaneous insertion of a proximal
humeral locking plate: an anatomic study. Injury 2007;38:206-211.
PJ, Clayson PE, Costenoble VH. Transitory percutaneous pinning in
fractures of the proximal humerus. J Shoulder Elbow Surg 1999;8:569-573.
JW, Cuomo F, Hill JD, et al. The difficult proximal humerus fracture:
tips and techniques to avoid complications and improve results. Instr
Course Lect 2007; 56:45-57.
K, Hamada J, Ohno W, et al. Surgical anatomy of multipart fractures of
the proximal humerus. J Shoulder Elbow Surg 2002;11:421-427.
MW, Cofield RH. Prosthetic arthroplasty for fractures and
fracture-dislocations of the proximal humerus. Clin Orthop Relat Res
1983;116-128.
MJ, Apreleva M, von Stechow D, et al. The cortical thickness of the
proximal humeral diaphysis predicts bone mineral density of the
proximal humerus. J Bone Joint Surg Br 2003;85B:611-617.
MJ, Bouxsein ML, Zurakowski D, et al. Three-dimensional distribution of
bone density in the proximal humerus. Calcif Tissue Int 2003;73:531-536.
MJ, Lehtinen J, Zurakowski D, et al. Proximal humeral fractures:
regional differences in bone mineral density of the humeral head affect
the fixation strength of cancellous screws. J Shoulder Elbow Surg
2006;15:620-624.
E, Peraldi P, Naouri JF, et al. [Four-part valgus impacted fractures of
the upper extremity of humerus: ilium graft reconstruction. Apropos of
8 cases]. Rev Chir Orthop Reparatrice Appar Mot 1996;82:658-662.
J, Thilak J, Mahajan CV. Arthroscopic treatment of acute traumatic
posterior glenohumeral dislocation and anatomic neck fracture.
Arthroscopy 2006;22:676-672.
CP, Tavy DL, Coene LN, et al. Electromyographic findings in shoulder
dislocations and fractures of the proximal humerus: comparison with
clinical neurological examination. Clin Neurol Neurosurg 1999;101:86-91.
G, Badet R, Nove-Josserand L, et al. Nonunions of the surgical neck of
the humerus: surgical treatment with an intramedullary bone peg,
internal fixation, and cancellous bone grafting. J Shoulder Elbow Surg
1996;5:161-168.
S, Reindl R, Harvey E, et al. Biomechanical comparison of a unique
locking plate versus a standard plate for internal fixation of proximal
humerus fractures in a cadaveric model. Clin Biomech Bristol Avon
2006;21:1027-1031.
DM, Bratton DR, Ciccone WJ, et al. Locking plates improve torsional
resistance in the stabilization of three-part proximal humeral
fractures. J Shoulder Elbow Surg 2006;15:239-243.
AJ, Roolker W, Patt TW, et al. Open reduction and internal fixation of
threeand four-part fractures of the proximal part of the humerus. J
Bone Joint Surg Am 2002;84A:1919-1925.
GR Jr, Copley LA, Iannotti JP, et al. The influence of intramedullary
fixation on figure-of-eight wiring for surgical neck fractures of the
proximal humerus: a biomechanical comparison. J Shoulder Elbow Surg
1997;6:423-428.
GR Jr, Wong KL. Two-part and three-part fractures: open reduction and
internal fixation versus closed reduction and percutaneous pinning.
Orthop Clin North Am 2000;31:1-21.
MA, Butters KP, Rockwood CA Jr. The posterior deltoid-splitting
approach to the shoulder. Clin Orthop Relat Res 1993;92-98.
MA, Jensen KL, Agarwal A, et al. Fracture-dislocation of the proximal
part of the humerus with retroperitoneal displacement of the humeral
head. A case report. J Bone Joint Surg Am 1997;79A:763-766.
RL, Kim DY, Arredondo J. Periprosthetic humeral fractures: management
and classification. J Shoulder Elbow Surg 1999;8:590-594.
M, Briot J, Pedrono A, et al. Age- and gender-related distribution of
bone tissue of osteoporotic humeral head using computed tomography. J
Shoulder Elbow Surg 2007;16:596-602.
KH. Helical plate fixation for treatment of comminuted fractures of the
proximal and middle one third of the humerus. Injury 2005;36:75-80.
Z, Goldberg I. Inferior subluxation of the humeral head after injury to
the shoulder. A brief note. J Bone Joint Surg Am 1989;71A:751-753.
TB, Wallace WA. Conservative treatment of fractures and
fracture-dislocations of the upper end of the humerus. J Bone Joint
Surg Br 1985;67B:373-377.
M, Weishaupt D, Jost B, et al. MR imaging for traumatic tears of the
rotator cuff: high prevalence of greater tuberosity fractures and
subscapularis tendon tears. AJR Am J Roentgenol 1999;172:463-467.
B, Poigenfurst J, Pezzei C, et al. Flexible intramedullary pins in the
treatment of unstable proximal humeral fractures. Injury 1991;22:60-62.
B, Poigenfurst J. [Treatment of unstable fractures of the proximal end
of the humerus using elastic curved intramedullary wires].
Unfallchirurgie 1987;13:72-81.
JD, Flugstad DL, Teitz CC, et al. Axillary artery injury as a
complication of proximal humeral fractures. Two case reports and a
review of the literature. Clin Orthop Relat Res 1984;234-237.
K, Ahrengart L, Sperber A, et al. Treatment of displaced proximal
humeral fractures in elderly patients. J Bone Joint Surg Br
1997;79B:412-417.
K, Wallace WA, Frostick S, et al. Outcomes in hemiarthroplasty for
three and four part fractures of the proximal humerus. J Shoulder Elbow
Surg 1998;7:85-89.
