The Optic Nerve
tested by examining the various modalities of vision: the visual
acuity, the visual fields (VFs), and special components of vision, such
as color vision and day and night vision. The optic nerve is the one
cranial nerve that can be visualized directly, and no neurologic, or
indeed general, physical examination is complete without an
ophthalmoscopic inspection of the optic disc and the retina.
testing acuity and color vision it is important to occlude the eye not
being tested. Before performing the optic nerve examination, look for
local ocular abnormalities such as cataract, conjunctival irritation,
corneal scarring or opacity, iritis, foreign bodies, photophobia, arcus
senilis, glaucoma, or an ocular prosthesis. The presence of a
unilateral arcus corneae with ipsilateral carotid disease has been
reported. In Wilson disease (hepatolenticular degeneration) a
yellowish-orange brown coloration 1 mm to 3 mm wide (Kayser-Fleischer
ring) may be seen around the rim of the cornea, more easily in
light-eyed individuals. It is due to copper deposition in the posterior
stroma and in Descemet’s membrane. It is best seen with a slit lamp.
Cataracts may be present in patients with myotonic dystrophy, certain
rare hereditary conditions with disturbed lipid or amino acid
metabolism, and in many other conditions.
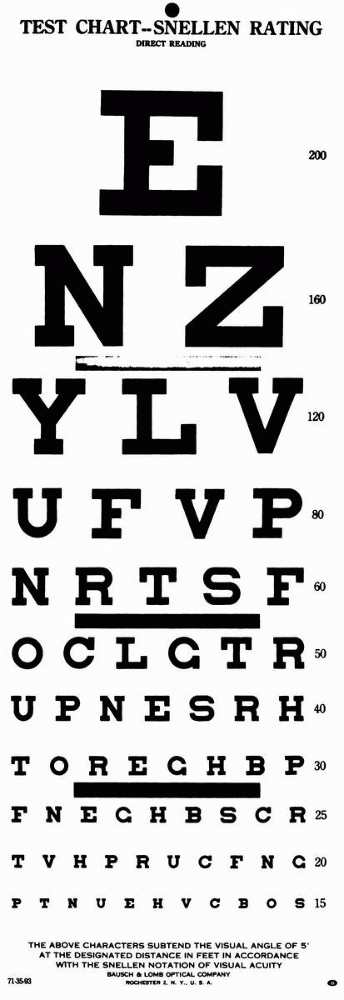
of letters, numbers, or figures that get progressively smaller, and can
be read at distances from 10 ft to 200 ft by normal individuals (Figure 9.1).
The difference between near and distance vision and between vision with
and without correction are points of primarily ophthalmological
interest. For neurologic purposes, only the patient’s best-corrected
visual acuity is pertinent. Refractive errors, media opacities, and
similar optometric problems are irrelevant. Acuity is always measured
using the patient’s accustomed correction.
Snellen chart is placed 20 ft from the patient; at that distance there
is relaxation of accommodation, and the light rays are nearly parallel.
The eyes are tested separately. In countries using the metric system,
the distance is usually given as 6 m. The ability to resolve test
characters (optotypes) approximately 1-in high at 20 ft is normal
(20/20 or 6/6) visual acuity. These characters subtend 5 minutes of
visual arc at the eye; the components of the characters (e.g., the
crossbar on the A) subtend 1 minute of arc. The acuity is the line
where more than half of the characters are accurately read. If the
patient can read the 20/30 line and two characters on the 20/25 line,
the notation is 20/30 + 2. By conventional notation, the distance from
the test chart, 20 or 6, is the numerator, and the distance at which
the smallest type read by
the
patient should be seen by a person with normal acuity is the
denominator. An acuity of 20/40 (6/12) means the individual must move
in to 20 ft to read letters a normal person can read at 40 ft. This
does not mean the patient’s acuity is one half of normal. In fact, an
individual with a distance acuity of 20/40 has only a 16.4% loss of
vision.
 |
|
FIGURE 9.1 • Snellen test chart.
|
have 20-ft eye lanes, testing is commonly done at a closer distance.
Neurologists frequently assess vision with a near card (see Toolkit).
Though examination of distance vision is preferable, the requisite
devices are generally not at hand. There are pocket cards designed for
testing at 6 ft, a convenient distance that usually
eliminates
the need for presbyopic correction (see Toolkit). Near vision is tested
with a near card, such as the Rosenbaum pocket vision screening card,
held at the near point (14 in or 35.5 cm). Good lighting is essential.
A penlight shone directly on the line being read is useful for bedside
testing.
distance may be shortened and the fraction adjusted. Ability to read
the line at 5 ft is vision of 5/200, equivalent to 20/800. Vision worse
than the measurable 20/800 is described as counts fingers (CF), hand
motion (HM), light perception (LP), or no light perception (NLP). The
average finger is approximately the same size as the 20/200 character,
so ability to count fingers at 5 ft is equivalent to an acuity of
20/800.
made to exclude refractive error by any available means. If the patient
has corrective lenses, they should be worn. In the absence of
correction, improvement of vision by looking through a pinhole suggests
impairment related to a refractive error. Commercial multi-pinhole
devices are available. A crude pinhole is included in the Toolkit. A
substitute can be made by making three or four holes with a pin in a 3
X 5 card in a circle about the size of a quarter. The multiple pinholes
help the patient locate one. The patient should then attempt to read
further down the acuity card through the pinhole. The pinhole permits
only central light rays to enter the eye. These are less likely to be
disrupted by refractive errors such as presbyopia and astigmatism. If a
pinhole was used, make some notation, such as 20/20 (ph). If the visual
impairment is due to a neurologic process, such as optic neuritis (ON),
vision will not improve with a pinhole. Under some circumstances, such
as with opacities in the media (e.g., cataract), vision may get worse
with pinhole.
present in about 3% to 4% of males. Disturbances of color vision may
also occur in neurologic conditions. Loss of color vision may precede
other visual deficits. Color deficits may be partial or total. Color
plates or pseudoisochromatic plates (Ishihara, Hardy-Ritter-Rand, or
similar) formally and quantitatively assess color vision. Having the
patient identify the colors in a fabric, such as a tie or a dress, can
provide a crude estimate. The Toolkit contains some screening plates
for color vision.
first. Desaturation to red, or red washout, describes a graying down or
loss of intensity of red. The bright red cap on a bottle of mydriatic
drops is a common test object. The patient compares the brightness or
redness in right versus left hemifields, temporal versus nasal
hemifields, or central versus peripheral fields. No right/left or
temporal/nasal desaturation to red occurs normally. Red does normally
look brighter in the center of the visual field than off center;
reversal of this pattern suggests impairment of central vision.
Patients may also compare the brightness or intensity of an examining
light in one eye versus the other. A diminution of brightness on one
side suggests optic nerve dysfunction; its significance is the same as
for red desaturation.
unfortunately, often omitted part of the neurologic examination. The VF
is the limit of peripheral vision, the area in which an object can be
seen while the eye remains fixed. Macular vision is sharp. Peripheral
images are not as distinct, and objects are more visible if they are
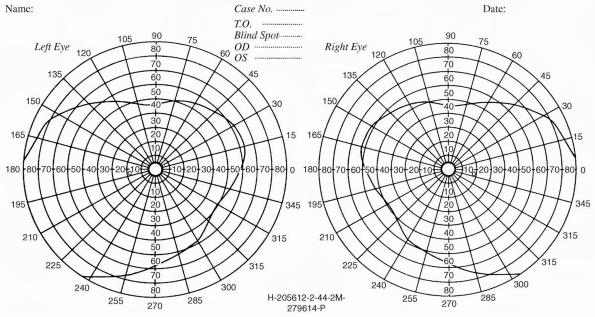
moving. The normal VF extends to 90 degrees to 100 degrees temporally,
about 60 degrees nasally, 50 degrees to 60 degrees superiorly, and 60
degrees to 75 degrees inferiorly. The field is wider in the inferior
and temporal quadrants than in the superior and nasal quadrants (Figure 9.2).
With binocular vision, the VFs of the two eyes overlap except for the
unpaired temporal crescent extending from 60 degrees to 90 degrees on
the horizontal meridian, which is seen by one eye only. The monocular
temporal crescent exists because of the anatomy of the retina. The
nasal retina extends farther forward, more peripherally, than the
temporal. This is the true reason that the temporal VF is more
expansive, not because the nose is blocking the nasal field.
 |
|
FIGURE 9.2 • The normal visual fields.
|
individual who is alert and cooperative and will maintain fixation.
Wandering of the eye impairs the evaluation. Crude assessment is
possible even in uncooperative patients if the target is interesting
enough (e.g., food or paper money). Fatigue and weakness may lengthen
the latency between perception of the test object and the response to
it, giving a false impression of VF deficit. Close cooperation, good
fixation, and adequate illumination are essential for mapping of the
blind spot and delineation of scotomas.
field evaluation. The time and energy expended on bedside confrontation
testing depends on the patient’s history and on the facilities
available for formal field testing with tangent screen (central 30
degrees) or perimetry (entire field). Even sophisticated confrontation
testing cannot approach the accuracy of formal fields.
the circumstances and done as superficially or as thoroughly as the
situation requires. Sophisticated bedside techniques can explore the
VFs in detail if circumstances warrant. If the patient has no specific
visual complaint, and if other aspects of the history and examination
do not suggest a field defect is likely, then a screening exam is
appropriate. This can be accomplished rapidly and with great
sensitivity using small amplitude finger movements in the far periphery
of the VF. Recall that the VFs extend temporally to 90+ degrees.
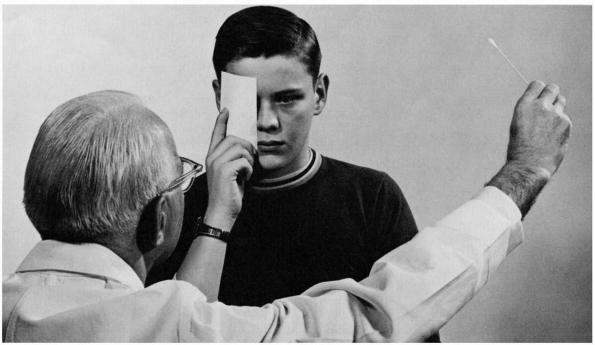
Extending elbows and index fingers, the examiner should position the
fingers nearly directly lateral to the lateral canthus at a distance of
about 24 in. Superficially, this appears to be a binocular examination,
but, properly placed, the finger targets are actually in the unpaired
monocular temporal crescent part of the visual field. With the targets
positioned, make a small amplitude flexion movement with the tip of one
index finger, perhaps 2 cm in amplitude. Have the patient “point to the
finger that moves.” This language is more efficient than attempting a
right-left verbal description where the patient’s and examiner’s rights
and lefts are reversed. Stimuli should be delivered in each upper
quadrant individually, then both together, and then similarly for the
lower quadrants. Including bilateral simultaneous stimuli is necessary
to detect subtle defects, which may be manifested only by extinction of
one stimulus on double simultaneous stimulation. This technique of
small finger movements in the far periphery in both upper and lower
quadrants is an excellent screen; when properly done, even binocularly,
this technique misses few VF defects. Always bear in mind that primary
ophthalmological disorders such as glaucoma, diabetic retinopathy, and
retinal detachment can also alter the visual fields.
 |
|
FIGURE 9.3 • Confrontation method of testing the visual fields.
|
could be expected to have a visual problem, higher-level testing is in
order. Examining monocularly, techniques include having the patient
assess the brightness and clarity of the examiner’s hands as they are
held in the right and left hemifields, in both upper and lower
quadrants, or having the patient count fingers fleetingly presented in
various parts of the field.
dimensions with the examiner’s, using various targets—still or moving
fingers, the head of a cotton swab, colored pinheads, or similar
objects. Positioning the patient and examiner at the same eye level,
and gazing eyeball to eyeball over an 18- to 24-in span, targets
introduced midway between and brought into the VF along various
meridians should appear to both people simultaneously in all parts of
the field except temporally, where the examiner must simply develop a
feel for the extent of a normal field (Figure 9.3).
money (the larger the denomination the better) makes a compelling
target. Even if the examiner has only a $1 bill, suggest to the patient
that it might be $100. The patient who can see will glance at or reach
for the object. Children may respond to keys (no jingling), candy, or
other visually interesting objects. Infants may turn the head and eyes
toward a diffuse light within a few days after birth. Moving a penlight
into the VF and noting when the patient blinks is sometimes useful.
Checking for blink to threat—the menace reflex—provides a crude
last-resort method. The examiner’s hand or fingers are brought in
rapidly from the side, as if to strike the patient or poke him in the
eye. The patient may wince, draw back, or blink. The threatening
movement should be deliberate enough to avoid stimulating the cornea
with an induced air current.
gaze at the examiner’s face and report any defects, such as a missing
or blurred nose. Having the patient survey a gridwork (Amsler grid)
while fixing on a central point is a sensitive method to detect
scotomas (see Toolkit). Probing the central field with a small white or
red object may detect moderate or large scotomas. With a cooperative
patient, one can estimate the size of the blind spot.
patient (i.e., right eye drawn on the right). This convention is
backwards from most things in clinical medicine, and violations of the
rule occur sufficiently often that labeling notations are prudent. When
confrontation fields are not
adequate
for the clinical circumstances, formal fields are done. These might
include tangent screen examination, kinetic perimetry, or computerized
automated static perimetry.
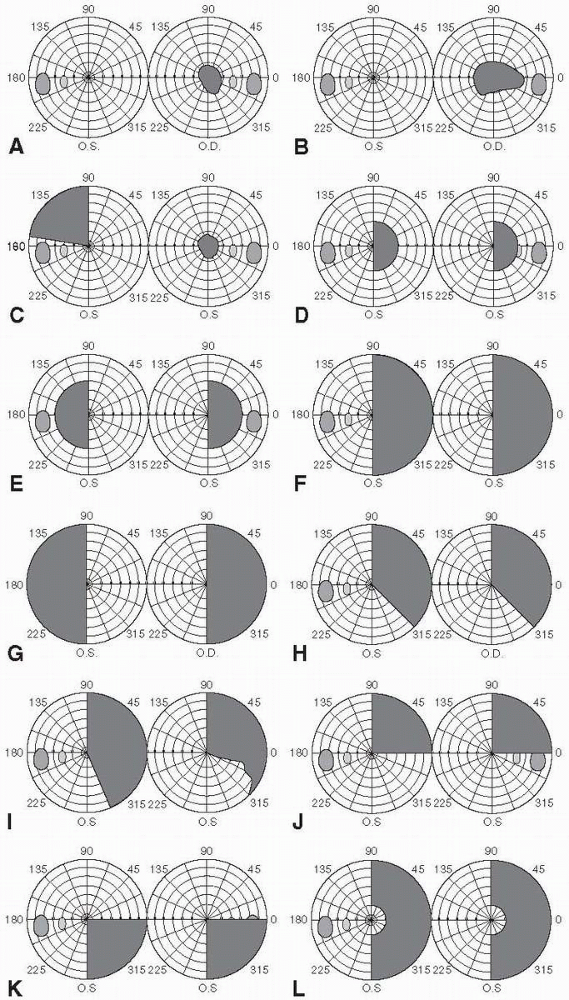
be divided into scotomas, hemianopias, altitudinal defects, and
concentric constriction or contraction of the fields. Figure 9.4
depicts some examples of different types of field defects. Because of
the anatomy and organization of the visual system, neurologic disorders
tend to produce straight-edged defects that respect either the
horizontal or vertical meridian, or have a characteristic shape because
of the arrangement of the nerve fiber layer (NFL). Respect of the
horizontal meridian may occur because of the horizontal temporal raphe
and the arching sweep of NFL axons above and below the macula. This
pattern is characteristic of optic nerve, optic disc, and NFL lesions.
The vascular supply of the retina consists of superior and inferior
branches of the central retinal artery, which supply the upper and
lower retina, respectively. Vascular disease characteristically causes
altitudinal field defects that are sharply demarcated horizontally. The
calcarine cortex is organized into a superior and an inferior bank, and
lesions involving only one bank may produce VF defects that respect the
horizontal meridian. The vertical meridian is respected because of the
division into nasal and temporal hemiretinas that occurs at the
chiasmal decussation and is maintained through the retrochiasmal visual
pathways.
with normal surrounding vision. With an absolute scotoma, there is no
visual function within the scotoma to testing with all sizes and colors
of objects. With a relative scotoma, visual function is depressed but
not absent; smaller objects and colored objects are more likely to
detect the abnormality. A positive scotoma causes blackness or a sense
of blockage of vision, as though an object were interposed; it suggests
disease of the retina, especially the macula or choroid. Positive
scotomas are often due to exudate or hemorrhage involving the retina or
opacity in the media. A negative scotoma is an absence of vision, a
blank spot as if part of the field had been erased; it suggests optic
nerve disease but can occur with lesions more posteriorly. With a
negative scotoma the defect may not be perceived until a VF examination
is done.
testing using small objects and carefully exploring the central fields,
but they are best demonstrated by the use of the tangent screen. The
physiologic blind spot is a scotoma corresponding to the optic nerve
head, which contains no rods or cones and is blind to all visual
impressions. The physiologic blind spot is situated 15 degrees lateral
to and just below the center of fixation because the disc lies nasal to
the macula and the blind spot is projected into the temporal field. The
blind spot is enlarged in papilledema and ON.
A central scotoma involves the fixation point and is seen in macular or
optic nerve disease. It is typical for ON but can occur in vascular and
compressive lesions (Figure 9.4A). A
paracentral scotoma involves the areas adjacent to the fixation point,
and it has the same implications as for a central scotoma. A
cecocentral scotoma extends from the blind spot to fixation. It is
usually accompanied by loss of all central vision with preservation of
a small amount of peripheral vision, and it strongly suggests optic
nerve disease (Figure 9.4B and Figure 9.5).
Central, paracentral, and cecocentral scotomas are all suggestive of a
process involving the papillomacular bundle. Any scotoma involving the
blind spot implies optic neuropathy.
the blind spot, usually due to optic neuropathy with the brunt of
damage falling on the fibers forming the superior and inferior nerve
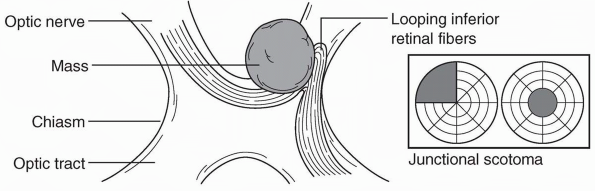
fiber layer arcades. A junctional scotoma is an optic nerve defect in
one eye (central, paracentral, or cecocentral scotoma) and a superior
temporal defect in the opposite eye. This is due to a lesion (usually a
mass) that involves one optic nerve close to the chiasm, which damages
the inferior nasal fibers from the opposite eye (Wilbrand’s knee) as
they loop forward into the proximal optic
nerve on the side of the lesion (Figure 9.6 and Figure 9.4C). The temporal VF defect in the contralateral eye may be subtle and easily missed.
 |
|
FIGURE 9.4 • Types of visual field defects. A. Central scotoma B. Cecocentral scotoma C. Junctional scotoma D. Homonymous scotomas E. Heteronymous scotomas F. Right homonymous hemianopia G. Bitemporal hemianopia H. Congruous right homonymous hemianopia I. Incongruous right homonymous hemianopia J. Right superior quadrantopia (“pie in the sky”) K. Right inferior quadrantopia L. Macular-sparing right homonymous hemianopia.
|
 |
|
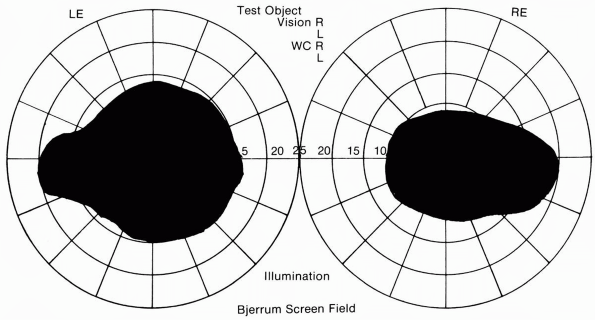
FIGURE 9.5 • Bilateral cecocentral scotomas in a patient with bilateral optic neuritis.
|
retina or optic nerve, they may also be caused by cerebral lesions.
Occipital pole lesions primarily affecting the macular area can produce
contralateral homonymous hemianopic scotomas (Figure 9.4D).
Since the bulk of fibers in the chiasm come from the macula, early
compression may preferentially affect central vision producing
bitemporal heteronymous paracentral scotomas (Figure 9.4E); with progression of the lesion, a full blown bitemporal hemianopia will appear (Figure 9.4G).
of each eye; hemianopic defects do not cross the vertical meridian.
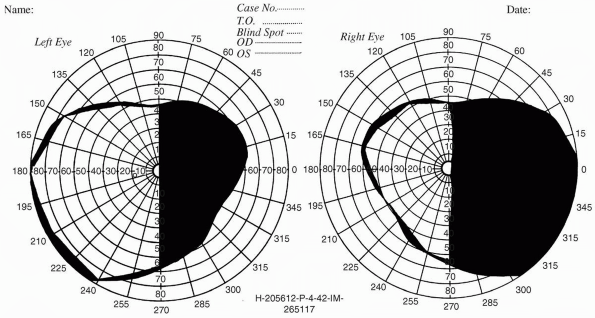
Hemianopias may be homonymous or heteronymous. A homonymous hemianopia
causes impaired vision in corresponding halves of each eye (e.g., a
right homonymous hemianopia is a defect in the right half of each eye).
Homonymous hemianopias are caused by lesions posterior to the optic
chiasm, with interruption of the fibers from the temporal half of the
ipsilateral retina and the nasal half of the contralateral retina.
Vision is lost in the ipsilateral nasal field and the contralateral
temporal field (Figure 9.7). A heteronymous hemianopia is impaired
vision in opposite halves of each eye (e.g., the right half in one eye
and the left half in the other). Unilateral homonymous hemianopias,
even those with macular splitting, do not affect visual acuity.
Patients can read normally with the preserved half of the macula, but
those with left-sided hemianopias may have trouble finding the line to
be read. Occasionally patients with homonymous hemianopia will read
only half of the line on the acuity chart.
 |
|
FIGURE 9.6 • A mass impinging on the optic nerve at its junction with the chiasm, producing a junctional scotoma.
|
 |
|
FIGURE 9.7 • Macular splitting right homonymous hemianopia in a patient with a neoplasm of the left occipital lobe.
|
If incomplete it may be congruous or incongruous. A congruous
hemianopia shows similarly shaped defects in each eye (Figure 9.4H).
The closer the optic radiations get to the occipital lobe, the closer
lie corresponding visual fibers from the two eyes. The more congruous
the field defect, the more posterior the lesion is likely to be. An
incongruous hemianopia is differently shaped defects in the two eyes (Figure 9.4I).
The more incongruous the defect, the more anterior the lesion. The most
incongruous hemianopias occur with optic tract and lateral geniculate
lesions. With a complete hemianopia, congruity cannot be assessed; the
only localization possible is to identify the lesion as contralateral
and retrochiasmal. A superior quadrantopsia implies a lesion in the
temporal lobe affecting Meyer’s loop (inferior retinal fibers): “pie in
the sky” (Figure 9.4J). An inferior quadrantopsia implies a parietal lobe lesion affecting superior retinal fibers (Figure 9.4K). A macular-sparing hemianopia is one that spares the area immediately around fixation; it implies an occipital lobe lesion (Figure 9.4L). The explanation for macular sparing remains unclear.
include partial or irregular defects in one or both of the hemifields,
relative rather than absolute loss of vision, an inability to localize
the visual stimulus, and hemianopia only for objects of a certain color
(hemiachromatopia). Extinction (visual inattention) is hemianopic
suppression of the visual stimulus in the involved hemifield when
bilateral simultaneous stimuli are delivered. Visual extinction is most
characteristic of lesions involving the nondominant parietal lobe.
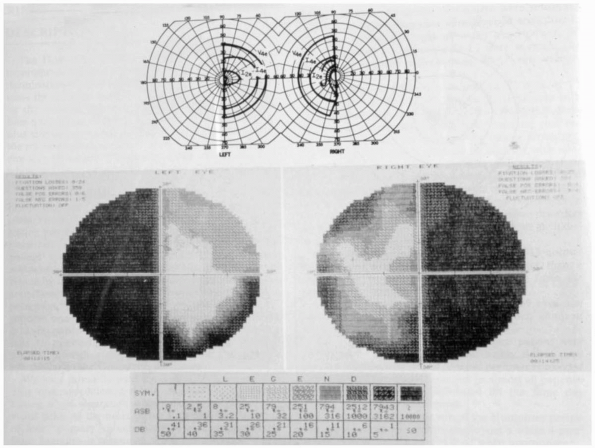
rarely are they binasal. A bitemporal hemianopia is usually due to
chiasmatic disease, such as a pituitary tumor growing up out of the
sella tursica and pressing on the underside of the chiasm (Figure 9.8).
Bitemporal field defects can usually be detected earliest by
demonstrating bitemporal desaturation to red. Because of the anterior
inferior position of decussating inferior nasal fibers, lesions
impinging from below produce
upper temporal field defects, which evolve into a bitemporal hemianopia (Figure 9.9).
Lesions encroaching from above tend to cause inferior temporal defects
initially. The defect will be first and worst in the upper quadrants
with infrachiasmatic masses (e.g., pituitary adenoma), and it will be
first and worst in the lower quadrants with suprachiasmatic masses
(e.g., craniopharyngioma). The most common cause of bitemporal
hemianopia is a pituitary adenoma; occasionally it results from other
parasellar or suprasellar lesions such as meningioma and
craniopharyngioma, as well as glioma of the optic chiasm, aneurysms,
trauma, and hydrocephalus.
 |
|
FIGURE 9.8
• (Top) Visual field performed on a Goldmann perimeter in a patient with a chiasmal lesion. (Bottom) Humphrey perimeter field in the same patient. (From Beck RW, Bergstrom TJ, Lichter PR. A clinical comparison of visual field testing with a new automated perimeter, the Humphrey Field Analyzer, and the Goldmann perimeter. Ophthalmology 1985; 92:77-82. Used with permission.) |
upper or lower half of vision, usually in one eye, and usually due to
retinal vascular disease (central retinal artery or branch occlusion or
anterior ischemic optic neuropathy). A partial altitudinal defect may
approximate a quadrantopsia. Altitudinal defects do not cross the
horizontal meridian.
of the range of vision, which may affect one or all parts of the
periphery. Constriction may be regular or irregular, concentric or
eccentric, temporal or nasal, and upper or lower. Symmetric concentric
contraction is most frequent and is characterized by a more or less
even, progressive reduction in field diameter through all meridians.
Concentric constriction of the VFs may occur with optic atrophy,
especially secondary to papilledema or late glaucoma, or with retinal
disease, especially retinitis pigmentosa. Diffuse depression is the
static perimeter equivalent of constriction on kinetic perimetry.
Concentric constriction of the fields is sometimes seen in hysteria. A
suspicious finding is when the fields fail to enlarge as expected with
testing at increasing distance (tubular fields).
 |
|
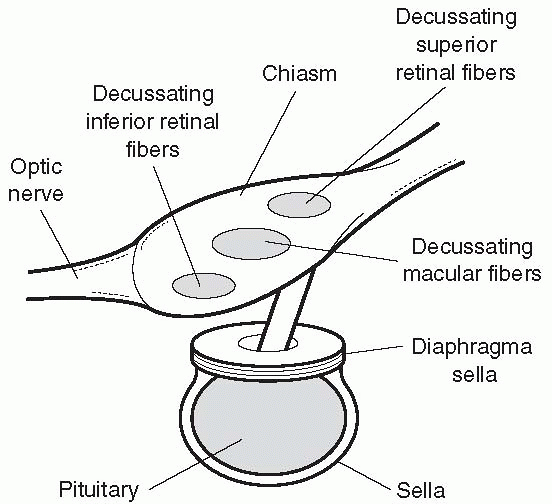
FIGURE 9.9
• The macular fibers decussate as a separate compact bundle, inferior retinal (superior visual field) fibers cross inferiorly, and superior retinal (inferior visual field) fibers superiorly. Masses impinging from below (e.g., pituitary adenoma) tend to cause early defects in the superior temporal fields; masses impinging from above (e.g., craniopharyngioma) tend to cause early defects in the inferior temporal fields. |
one-eyed Eskimo peering into a dark igloo from the entry way with a
flashlight. Only a narrow sector of the posterior pole is visible, and
there is no stereopsis. Pupil dilation significantly increases the
field of view. Indirect ophthalmoscopy, used by ophthalmologists, can
stereoscopically view almost the entire vista of the fundus. New
PanOptic direct ophthalmoscopes (Welch-Allyn) give the advantage of a
broader view but still reveal only the posterior pole. In the
neurologic examination, the areas of primary concern are the disc, the
macula, and the arteries. The disc is normally round or a vertically
oriented slight oval. The nasal margin is normally slightly blurred
compared to the temporal. The disc consists of a peripheral
neuroretinal rim and a central cup. The neuroretinal rim consists of
axons streaming from the retina to enter the optic nerve. The
physiologic cup is a slight depression in the center of the disc that
is less pinkish than the rim and shows a faint latticework due to the
underlying lamina cribrosa. The rim is elevated slightly above the cup.
To locate the disc, a helpful technique is to find a retinal blood
vessel, focus on it, and then follow it to the disc. The myelinated
axons making up its substance render the normal optic disc yellowish
white. It is paler temporally where the papillomacular bundle enters.
The normal disc lies flat and well demarcated against the surrounding
retina, with arteries and veins crossing the margins and capillaries
staining the surface a faint pink. Varying amounts of pigmentation are
present in the retina near the temporal border of the disc, especially
in dark-skinned persons. At times a pigment ring may completely
surround the disc. The macula is a dark area that lies about 2 disc
diameters temporal to and slightly below the disc.
into prechiasmal, chiasmal, and retrochiasmal. Disease in each of these
locations has characteristic features that usually permit its
localization. The etiologic processes affecting these different
segments of the afferent visual system are quite different. As a
generalization, prechiasmal lesions cause monocular visual loss;
impaired color perception; a central, paracentral, or cecocentral VF
defect; and an afferent pupillary defect (APD).
The
disc may or may not appear abnormal depending on the exact location of
the lesion. Chiasmal lesions cause heteronymous VF defects, most often
bitemporal hemianopia, with preservation of visual acuity and color
perception and a normal appearing optic disc. Retrochiasmal lesions
cause a contralateral homonymous hemianopia and have no effect on
acuity or disc appearance. There is usually no effect on color vision,
but some central lesions may cause achromatopsia. A summary of the
features of disease involving the macula, optic nerve, chiasm, optic
tract, lateral geniculate body (LGB), optic radiations, and calcarine
cortex can be found in Table 9.1.
can be divided into those that affect the disc (papillopathy) and those
that affect the retrobulbar segment between the globe and the chiasm.
The macula gives rise to the majority of the fibers in the optic nerve,
and disease of the macula itself can cause a clinical picture that is
at times difficult to distinguish from optic neuropathy. Common causes
of maculopathy include age-related macular degeneration and central
serous retinopathy (Table 9.1).
variety of circumstances. The disc may change color—to abnormally pale
in optic atrophy or to abnormally red with disc edema. The margins may
become obscured because of disc edema or the presence of anomalies.
Edema of the disc is nonspecific. It may reflect increased intracranial
pressure, or it may occur because of optic nerve inflammation,
ischemia, or other local processes. By convention, disc swelling due to
increased intracranial pressure is referred to as papilledema; under
all other circumstances, the noncommittal terms disc edema or disc
swelling are preferred. Visual function provides a critical clue to the
nature of disc abnormalities. Patients with acute papilledema and those
with disc anomalies have normal visual acuity, visual fields, and color
perception. Impairment of these functions is the rule in patients
suffering from optic neuropathies of any etiology. The first step in
evaluating a questionably abnormal disc is therefore a careful
assessment of vision.
optic nerves, which impairs axoplasmic flow and produces axonal edema
and an increased volume of axoplasm at the disc. The swollen axons
impair venous return from the retina, engorging first the capillaries
on the disc surface, then the retinal veins, and ultimately causing
splinter- and flame-shaped hemorrhages as well as cotton wool exudates
in the retinal nerve fiber layer. Further axonal swelling eventually
leads to elevation of the disc above the retinal surface.
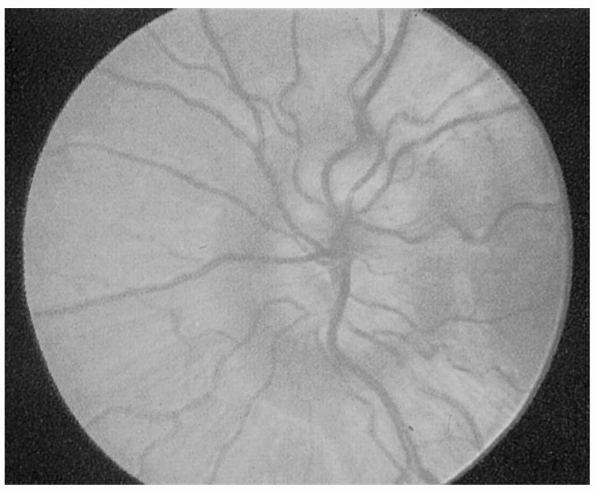
developed, chronic, and atrophic. Fully developed papilledema is
obvious, with elevation of the disc surface, humping of vessels
crossing the disc margin, obliteration of disc margins, peripapillary
hemorrhages, cotton wool exudates, engorged and tortuous retinal veins,
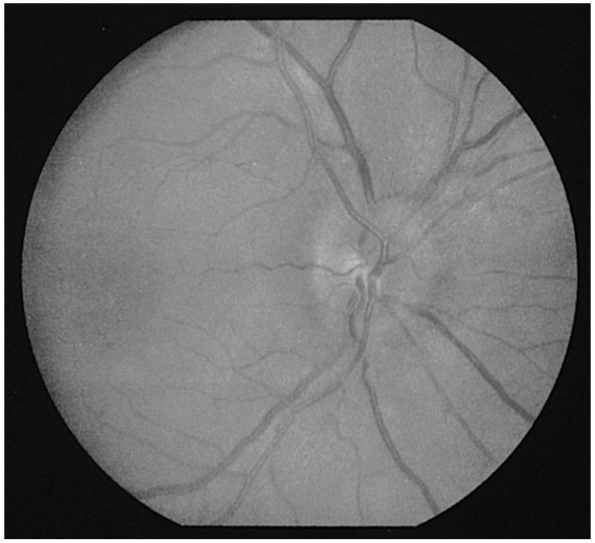
and marked disc hyperemia. The recognition of early papilledema is much
more problematic (Figure 9.10). Occasionally,
the only way to resolve the question of early papilledema is by serial
observation. The earliest change is loss of previously observed
spontaneous venous pulsations (SVP). The presence of SVPs indicates an
intracranial pressure less than approximately 200 mm H20.
However, since they are absent in 10% to 20% of normals, only the
disappearance of previously observed SVPs is clearly pathologic.
dilates the capillaries on the disc surface, transforming its normal
yellowish-pink color to fiery red. Blurring of the superior and
inferior margins evolves soon after. However, since these margins are
normally the least distinct areas of the disc, blurry margins alone are
not enough to diagnose papilledema. There is no alteration of the
physiologic cup with early papilledema. With further evolution, the
patient with early papilledema will
develop
diffuse disc edema, cup obscuration, hemorrhages, exudates, and venous
engorgement. Frank disc elevation then ensues as the fundus ripens into
fully developed papilledema (Figure 9.11).
In chronic papilledema, hemorrhages and exudates resolve and leave a
markedly swollen “champagne cork” disc bulging up from the plane of the
retina. If unrelieved, impaired axoplasmic flow eventually leads to
death of axons and visual impairment, which evolves into the stage of
atrophic papilledema, or secondary optic atrophy. Papilledema
ordinarily develops over days to weeks. With acutely increased
intracranial pressure due to subarachnoid or intracranial hemorrhage,
it may develop within hours. Measuring diopters of disc elevation
ophthalmoscopically has little utility.
|
TABLE 9.1 Clinical Characteristics of Acute Lesions Involving Different Parts of the Afferent Visual Pathway
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|
FIGURE 9.10 • Early papilledema.
|
 |
|
FIGURE 9.11 • Severe papilledema.
|
|
TABLE 9.2 Some Causes of Unilateral Disc Edema
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
or color vision. The blind spot may be enlarged, but VF testing is
otherwise normal. In patients who develop optic atrophy following
papilledema, the visual morbidity can be severe and may include
blindness.
papilledema occur when conditions primarily affecting the optic nerve
papilla cause disc edema. Papilledema is usually bilateral; other
causes of disc edema are often unilateral (Table 9.2).
Optic neuropathies generally cause marked visual impairment, including
loss of acuity, central or cecocentral scotoma, loss of color
perception, and an APD. Disease of the optic nerve head is usually due
to demyelination, ischemia, inflammation, or compression. There are
many causes of optic neuropathy; some of the more common conditions are
listed in Table 9.3.
disc changes of little or no clinical import. This circumstance arises
frequently when routine ophthalmoscopy unexpectedly reveals an abnormal
appearing disc in a patient with migraine or some seemingly benign
neurologic complaint. Such
patients
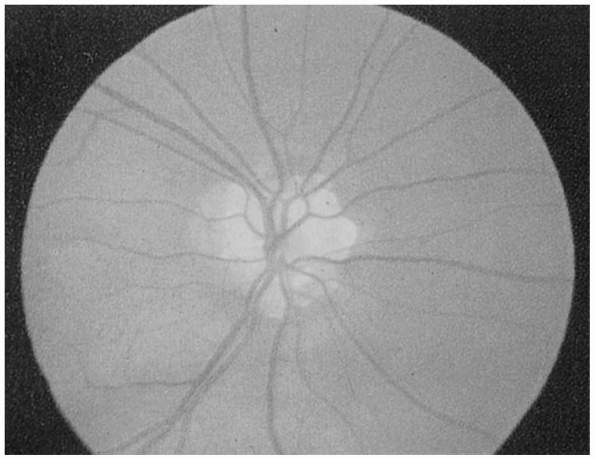
generally have normal vision and no visual complaints. Common causes of
pseudopapilledema include optic nerve drusen and myelinated nerve
fibers (Figures 9.12 and 9.13). Distinguishing pseudopapilledema from acquired disc edema can be difficult.
|
TABLE 9.3 Some Causes of Optic Neuropathy
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
 |
|
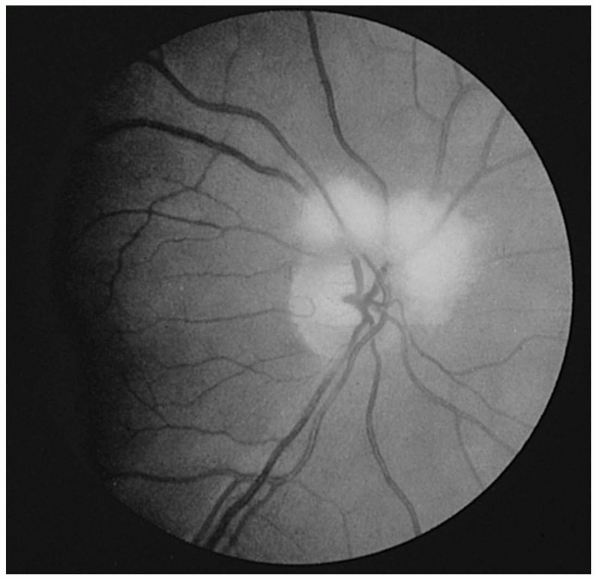
FIGURE 9.12 • Drusen of the optic nerve head simulating papilledema.
|
sharply demarcated from the surrounding retina, sometimes having a
punched-out appearance (Figure 9.14). The disc
margins stand out distinctly; the physiologic cup may be abnormally
prominent and extend to the margin of the disc. Loss of myelinated
axons
and their supporting capillaries with replacement by gliotic scar
produce the lack of color, which may vary from a dirty gray to a
blue-white color to stark white. An atrophic disc may appear
perceptibly smaller. Pallor of the temporal portion of the disc—a
classical finding in MS—may precede definite atrophy, but normal
physiologic temporal pallor makes this finding often equivocal.
 |
|
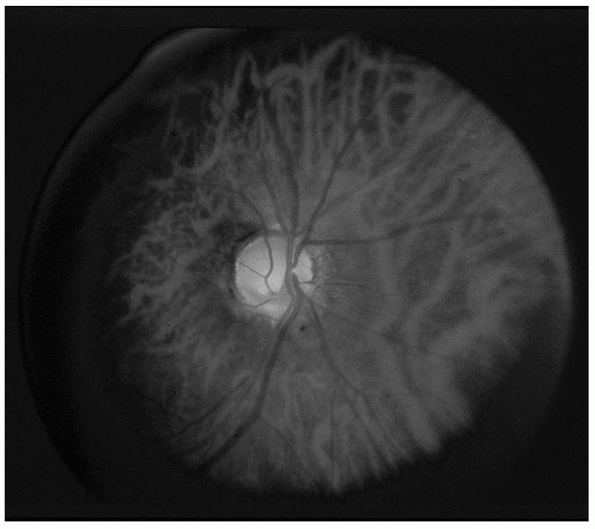
FIGURE 9.13 • Medullated nerve fibers.
|
 |
|
FIGURE 9.14 • Primary optic atrophy.
|
anterior ischemic optic neuropathy, or papilledema) and is then
referred to as secondary or consecutive optic atrophy. Primary optic
atrophy, appearing de novo, occurs as a heredofamilial condition (e.g.,
Leber hereditary optic neuropathy) or after toxic, metabolic,
nutritional, compressive, or glaucomatous insult to the nerve. Some
causes of optic atrophy are listed in Table 9.4. A patient may have disc edema in one eye and optic atrophy in the other eye (Foster Kennedy syndrome).
|
TABLE 9.4 Some Causes of Optic Atrophy
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
most of the diseases that affect the optic disc. The clinical picture
is similar except that there is no disc edema acutely, but optic
atrophy may follow later. When ON strikes the retrobulbar portion of
the nerve, marked visual impairment occurs but the disc appearance
remains normal, since the pathology is posterior to the papilla. Optic
papillopathy thus causes impaired vision and an abnormal disc;
retrobulbar optic neuropathy causes impaired vision and a normal disc;
and papilledema causes an abnormal disc but does not affect vision
acutely. A major difference between retrobulbar neuropathy and
papillopathy is the increased incidence of compression as an etiology
in the former.
gliomas, and carotid aneurysms are the lesions that commonly involve
the chiasm. Because the chiasm lies about a centimeter above the
diaphragma sella, visual system involvement indicates suprasellar
extension of a pituitary tumor and is a late, not an early,
manifestation of chiasmatic mass effect. Acuity, color vision, and
pupillary function are not affected unless there is optic nerve
involvement.
VF defects that respect the vertical meridian. Optic tract and LGB
lesions are rare, and characterized by incongruous homonymous
hemianopias. In geniculocalcarine pathway (optic radiation) lesions,
temporal lobe pathology typically produces contralateral superior
quadrantopias, or homonymous hemianopia worse in the upper quadrants;
and parietal lobe processes contralateral inferior quadrantopias, or
homonymous hemianopia worse in the lower quadrants (Figure 9.4).
The more posterior the lesion, the more congruous the defect. Occipital
lobe lesions cause contralateral homonymous hemianopias that are highly
congruous and tend to spare the macula. Hypotensive watershed
infarctions may cause contralateral homonymous paracentral scotomas due
to ischemia limited to the macular cortex (Figure 9.4D).
defects of cortical function in addition to the visual loss. Anton
syndrome is cortical blindness due to bilateral homonymous hemianopias,
with extreme visual impairment in which the patient is unaware of, and
denies the existence of, the deficit.
