Knee Dislocations
devastating complications secondary to damage to multiple soft-tissue
and stabilizing structures. Associated injuries may include the
cruciate ligaments, collateral ligaments, medial and lateral capsular
structures, menisci and articular cartilage, as well as neurovascular
injuries and compartment syndrome. Traditional nonoperative treatment
has resulted in poor outcomes. Surgical treatment remains controversial
with respect to timing of surgery, which structures to repair versus
reconstruct, and choice of grafts. We believe early surgical repair is
indicated in the vast majority of multiligament injuries that result
from knee dislocation.
undergo an extensive preoperative workup to discern the details of his
or her injury and to insure he or she is medically stable for surgery.
Inherent in this evaluation is detailed attention to factors that may
predicate emergent surgical intervention. Evaluation in the trauma bay
that reveals an open dislocation, irreducible dislocation, or arterial
injury requires an emergency trip to the operating room. In the case of
arterial injury, application of a joint-spanning external fixator
provides joint stability and protects the vascular graft. Recovery of
associated soft-tissue injury determines whether to perform later
reconstruction or repair of the ligaments. Following a vascular repair,
reconstructive procedures should be postponed until the vascular graft
has matured and is not likely to be compromised. After vascular
repairs, four-compartment fasciotomies are often required to combat
reperfusion injuries.
preexisting functional deficits are not usually candidates for surgical
repair and are often better treated with bracing and a functional
rehabilitation protocol. Other contraindications to surgery are an
active infection, displaced intra-articular or peri-articular fractures
of the femur or tibia, and advanced osteoarthritis of the knee.
dislocation begins in the emergency room and requires a very detailed
physical exam as well as specific radiological exams, including
angiography, to elucidate the extent of the injury. Early vascular
surgery consultation is recommended because a normal physical exam does
not rule out vascular injury in the acute phase. The physician must
also maintain a high index of suspicion for compartment syndrome. The
physical exam in these patients can be difficult and inaccurate
secondary to pain. Prompt reduction of the dislocation by traction and
countertraction under conscious sedation is essential. Appropriate
films should be obtained to rule out fractures and to ensure reduction.
Stress radiographs may occasionally be used to evaluate varus and
valgus instability. Due to the limits of the physical exam, when the
patient is medically stable, a magnetic resonance imaging (MRI) scan is
necessary to assess the extent of injury and to assist in surgical
planning. Soft tissue swelling and generalized edema may hinder the
quality of the MRI obtained.
management of knee dislocation and include timing of surgery: what
structures to repair or reconstruct, the choice of grafts, and the
surgical techniques. Recent publications have shown that operative
treatment gives better results than nonoperative treatment. Although
there are numerous approaches and techniques for these difficult cases,
this chapter will present our experience and approach to the
knee-dislocation patient.
acute ligamentous repair and reconstruction within 3 weeks of injury
have done better than patients reconstructed after 3 weeks, as
determined by Lysholm and Knee Outcome Survey Activities of Daily
Living scores. However, in patients with severe, life-threatening or
arterial injuries, or those with open dislocations, ligamentous
reconstruction is delayed until soft-tissue swelling has subsided and
the patient’s condition has improved.
tissues remains controversial. We believe that the repair and
reconstruction of all associated ligamentous and meniscal injuries
should be undertaken in patients having surgery. The majority of
injuries to the cruciate ligaments are intrasubstance and do not
respond favorably to primary repair. An exception to this is when large
bony fragments from the tibial insertions of the anterior cruciate
ligament (ACL) or posterior cruciate ligament (PCL) are avulsed. In
these situations we advocate primary repair by passing large
nonabsorbable sutures into the bony fragment and through the bone
tunnels in the tibia. In all other situations, we reconstruct ACL and
PCL injuries. At our institution we attempt to preserve specific
bundles of the PCL that are not injured. In one third of cases, the
anterolateral bundle is ruptured, but the meniscofemoral ligament and
the posteromedial bundle remain intact. In these cases we do a single
bundle reconstruction of the anterolateral bundle.
injuries, we often acutely repair (less than 3 weeks after injury) the
injured structures if the tissue quality is adequate. Depending on the
stability of the repair, augmentation is often necessary. Chronic
injuries (more than 3 weeks after injury) tend to be limited by scar
formation and soft-tissue contracture and require reconstruction.
traumatized knee, and decrease donor site morbidity, we advocate the
use of allografts over autografts in multiple-ligament reconstruction
surgery. Inherent in this choice of allografts is the risk of
transmissible disease with allografts. This must be discussed with the
patient prior to the procedure.
surgeon, anesthesiologist, and the patient, taking medical
co-morbidities, age, and prior history with anesthesia into
consideration.
General
anesthesia with concomitant IV sedation is most commonly performed.
Preoperative femoral and/or sciatic nerve blocks are routinely
performed as an adjuvant for postoperative pain relief. A Foley
catheter may be placed intraoperatively to monitor fluid shifts during
the procedure.
table. The goal is to allow up to 80 to 90 degrees of static flexion of
the knee to be maintained without manual assistance. To do so, a small
bump is placed under the patients leg just distal to the greater
trochanter with a lateral post placed at the same level. A sterile bump
is wedged between the post and the thigh. To maintain flexion, the heel
rests on a 4.5 kg sandbag that has been taped to the bed and prevents
extension of the leg (Fig. 25.1). Due to the length of time these cases require, we do not use a tourniquet.
table, a complete ligamentous exam, including anterior and posterior
drawer tests, pivot shift, dial test, and posterior drawer with
external rotation, is performed. These exam findings are corroborated
with MRI findings to establish the proper surgical approach to address
the anticipated pathologies.
Gerdy’s tubercle, the fibular head, and medial and lateral joint lines
are marked. Special care is taken to palpate and identify the peroneal
nerve as it courses around the fibular neck. The dorsalis pedal pulse
is palpated and marked for continual monitoring. The standard
anterolateral arthroscopic portal is placed just adjacent to the
lateral border of the patella tendon, above the joint line. The
anteromedial portal is established at the same level, approximately 1
cm medial to the patellar tendon. Additionally, a superolateral outflow
portal is established in standard fashion, superior to the patella and
posterior to the quadriceps muscle. The posteromedial portal is often
required to address the tibial insertion of the PCL, and it is
established intraoperatively using an inside-out technique with the aid
of a 70-degree arthroscope.
that require repair. Our standard ACL/PCL tibial tunnel is marked 3 cm
longitudinally over the anteromedial proximal tibia, 2 cm distal to the
joint line and 2 cm medial to the tibial tubercle. The incision for the
PCL femoral tunnel is marked 2 cm medial to the medial articular
surface of the trochlea in the subvastus interval. If the injury has an
associated medial component, the incision for the tibial tunnels is
extended proximally in curvilinear fashion to the medial epicondyle.
The incision
for
lateral and posterolateral injuries is made, with the knee in flexion,
in curvilinear fashion and extends proximally from the lateral
epicondyle distally to a location midway between Gerdy’s tubercle and
the fibular head (see Fig. 25.1)
The proximal portion of this incision parallels the plane between the
biceps femoris tendon and the iliotibial band. The skin incisions are
injected preoperatively with 0.25% Marcaine with epinephrine
(1:100,000).
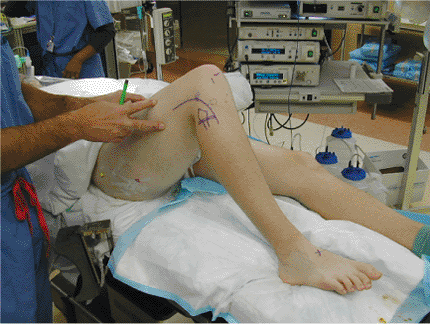 |
|
Figure 25.1. Set-up, surface anatomy, and skin incisions with a lateral-sided injury.
|
injury involving multiple ligaments, most of which are surgeon
dependent. The timing of surgery, extent of injury, experience of
surgeon, and availability of allograft all factor into the selection
process. The inherent advantages to autograft are offset in these
complex cases by a choice of allograft, which will provide less
donor-site morbidity and decreased operative time. For the ACL graft we
currently prefer to use allograft bone-patellar tendon-bone graft.
Soft-tissue allograft such as tibialis anterior is also a viable
choice. For the PCL, we prefer to use Achilles tendon allograft because
of its length, its girth, and the existence of the calcaneal bone plug
for femoral fixation (Fig. 25.2). The lateral
collateral ligament (LCL) is usually reconstructed with an Achilles
tendon allograft with a 7- to 8-mm calcaneal bone plug, which will be
fixed in a proximal fibular-bone tunnel at the native LCL insertion
site. The posterolateral corner is usually reconstructed with either a
tibialis anterior allograft or a semitendinosus autograft.
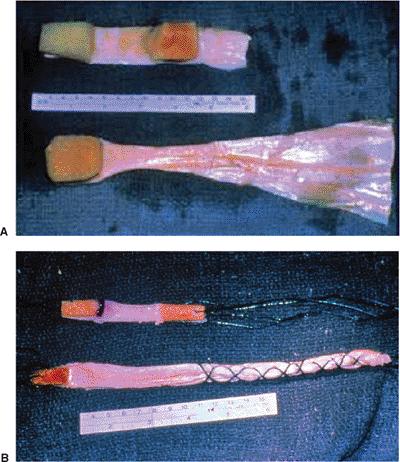 |
|
Figure 25.2. Bone–patellar tendon–bone allograft for ACL reconstruction and Achilles tendon allograft for PCL reconstruction: (A) before preparation and (B) after preparation.
|
gravity inflow irrigation with a superolateral outflow portal rather
than a pump. The arthroscopic technique should be abandoned in favor of
an open approach should extravasation be noted or a compartment
syndrome suspected. A 30-degree arthroscope is introduced through the
anterolateral portal and a diagnostic arthroscopy is performed to
assess the integrity of the cruciate ligaments, the menisci, and the
articular cartilage. A 70-degree scope is now placed through the
anterolateral portal to establish the posteromedial portal, which will
be used as the working portal for the tibial insertion of the PCL.
Extreme caution should be exercised in this area to avoid extension of
the debridement beyond the capsule and thus causing injury to the
neurovascular structures, which reside approximately 1.5 cm posterior
to the tibial insertion of the PCL. The use of the 30- and 70-degree
arthroscopes through both the anterolateral and the posteromedial
portal will allow for excellent access, visualization, and
triangulization of the tibial PCL insertion site. Our attention is now
turned to the ACL. We attempt to preserve as much of the ACL footprint
as possible for vascular and pro-prioceptive considerations.
articular cartilage injuries. Peripheral tears will be repaired using
our preferred inside-out method, leaving the sutures to be secured with
the knee in 30 degrees of flexion after all other grafts have been
passed and secured. Central and irreparable tears are debrided back to
a stable rim at this time.
the more difficult of the two. With the aid of arthroscopic
visualization, a 15-mm offset PCL guide is set between 50 and 55
degrees and passed through the anteromedial portal with the tip of the
guide located in the most distal and lateral portion of the native PCL
insertion site (Fig. 25.3). A Kirschner (K)
wire is passed with a starting point approximately 3 to 4 cm distal to
the joint line, and an intraoperative fluoroscopy image is obtained.
This K wire is left in place and attention is turned to the ACL tunnel.
The ACL guide is set to 45 degrees and inserted through the
anteromedial portal. A 3/32-inch guide wire is inserted into the center
of the ACL footprint. A fluoroscopic image with the knee in full
extension is now obtained to assess the placement of the K wires. The
ACL wire should be just posterior to Blumenstaat’s line on the lateral,
and the PCL wire should be 2 to 3 cm distal to the ACL wire (Fig. 25.4).
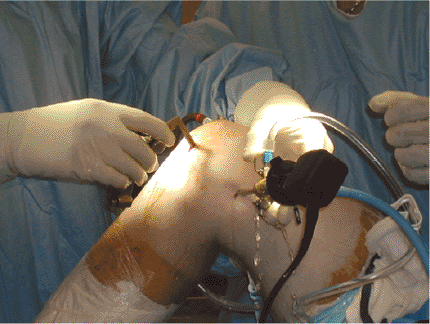 |
|
Figure 25.3. Clinical photograph demonstrating position of the arthroscope in the posteromedial portal while drilling the PCL tibial tunnel.
|
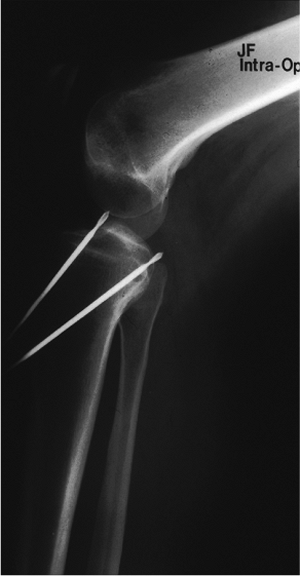 |
|
Figure 25.4. Radiographic confirmation of the ACL and PCL tibia-guide wire placement.
|
positioning, the PCL tibial tunnel is drilled. A curette is placed
directly on top of the guide wire, and with direct visualization via a
30-degree arthroscope in the anteromedial portal, a compaction drill
bit is passed. The drill is started with pneumatic power, but the
drilling is finished by hand after the initial cortex is breeched.
Maintaining meticulous attention to the visualization of the K wire
tip, we drill 1 mm less than the desired tunnel width. We then dilate,
by hand, in 0.5-mm increments. The same technique is used when
preparing the ACL tunnel. We desire to have at least a 2-cm bone bridge
between the ACL and PCL tibial tunnels.
tunnels. For a single bundle PCL reconstruction, a K wire is passed
from the anterolateral portal to a point within the anterior portion of
the PCL femoral footprint, which is located approximately 5 to 6 mm
from the articular margin. This is overdrilled with a compaction drill
to a depth of approximately 25 to 35 mm. If a double-bundle PCL
reconstruction is being performed, the anterolateral tunnel is drilled
at 1 o’clock, approximately 5 to 6 mm off of the articular cartilage.
This allows for anterior placement of the tunnel. The tunnel for the
posteromedial graft is much smaller and is drilled in the posterior
footprint at approximately the 3 to 4 o’clock position, 1 cm posterior
to the anterolateral tunnel. The ACL femoral tunnel is created with the
knee hyperflexed. We use a medial portal approach, rather than
transtibial, to drill the femoral tunnel. This approach allows
positioning and angulation of the femoral tunnel to be independent of
the tibial-tunnel angle. In this method, a K wire is placed through the
anteromedial portal and into the center of the anatomic insertion of
the ACL, approximately 6 to 7 mm anterior to the over-the-top position
(at the 1:30 to 2:00 position for left knees or the 10:00 to 10:30
position for right knees). As with the PCL tunnel, the K wire is over
drilled with a compaction drill to a depth of 25 to 35 mm, and the hole
is expanded to the desired graft size (Fig. 25.5).
is passed, in retrograde fashion through the PCL tibial tunnel and
retrieved under arthroscopic visualization by a pituitary rongeur
inserted through the anterolateral portal. The no. 5 nonabsorbable
suture that was
placed
through the tibial bone block is now placed through the eyelet on the
wire that is external to the anterolateral portal. The other end of the
wire is then withdrawn from the tibial PCL tunnel in antegrade fashion
bringing the no. 5 suture and tibial portion of the PCL graft with it.
For the femoral side of the graft, the no. 5 suture, which had
previously been tagged and has remained outside of the anterolateral
portal, is place through the eyelet of a Beath pin. The Beath pin is
subsequently passed through the anterolateral portal into the PCL
femoral tunnel and out the anteromedial thigh. Under arthroscopic
visualization, tensioning the sutures and gentle guidance from an
arthroscopic probe are used to assist graft passage.
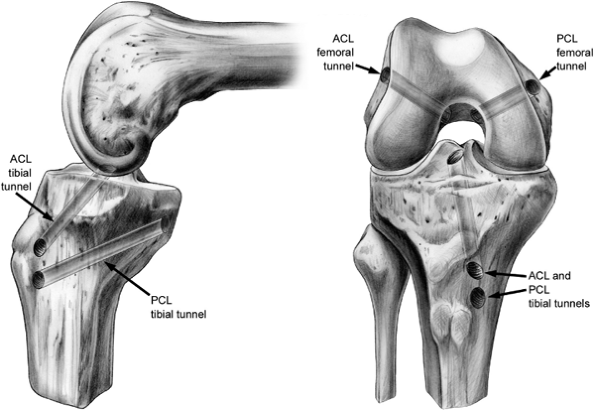 |
|
Figure 25.5. Schematic of ACL and PCL tibial and femoral tunnels.
|
pin with a no. 5 suture through the eyelet is passed through the medial
portal and out of the femoral tunnel to the anterolateral thigh. With
one end of the suture exiting the superolateral portal and one end
exiting from the medial portal, a pituitary rongeur is passed
retrograde through the tibial tunnel, and the no. 5 suture is withdrawn
from medial portal and passed out of the tibial ACL tunnel. This end of
the suture is now looped through the tagged femoral end of the ACL
graft, and the graft is passed with arthroscopic assistance as
described for the PCL graft. The femoral fixation of both grafts is now
performed, but tensioning of the tibial grafts is delayed until the end
of the case.
the PCL grafts. Depending on graft type and quality of bone,
interference screws or a 4.5-mm AO screw, used as a post, can be used.
between the posterior edge of the iliotibial band and the biceps
femoris, and to allow visualization of the LCL and popliteofibular
ligament (PFL) insertions (Fig. 25.6). If the
injuries to these structures are acute and the soft tissues allow,
primary repair can be attempted with a no. 2 braided, nonabsorbable
suture (Fig. 25.7). If reconstruction of the LCL is indicated, we prefer to use an
Achilles allograft, with imbrication of the native LCL by a whipstitch.
The tendinous portion of the allograft is secured to the LCL femoral
insertion via drill holes or suture anchors. The distal insertion of
the LCL on the fibula is dissected free, and a tunnel is drilled along
the longitudinal axis of the fibula. The calcaneal bone plug is secured
in this bony tunnel with a metal interference screw. It is tensioned
with the knee in 30 degrees of flexion (Fig. 25.8).
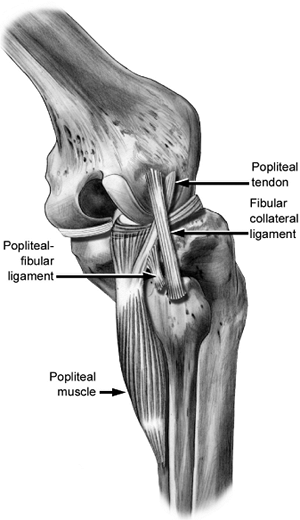 |
|
Figure 25.6.
Anatomic relationships of the lateral side of the knee. Popliteus tendon (PT). Lateral collateral ligament (LCL). Popliteofibular ligament (PFL). |
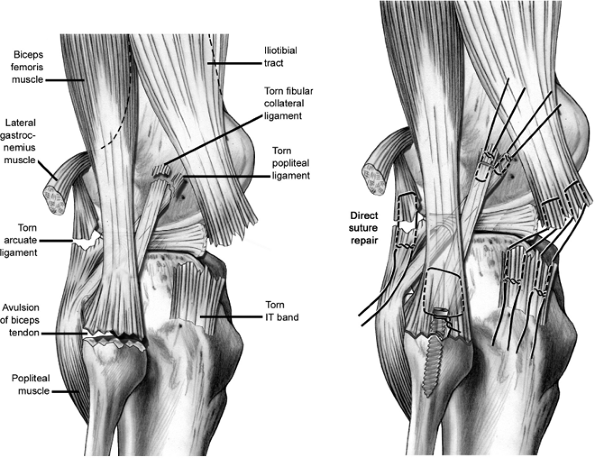 |
|
Figure 25.7. Direct repair of the lateral structures.
|
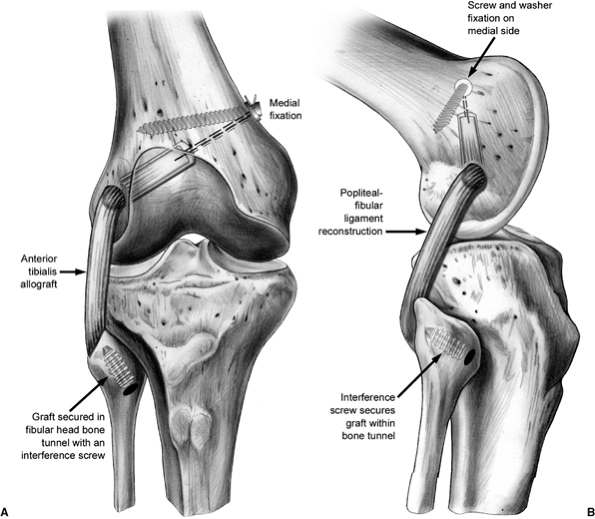
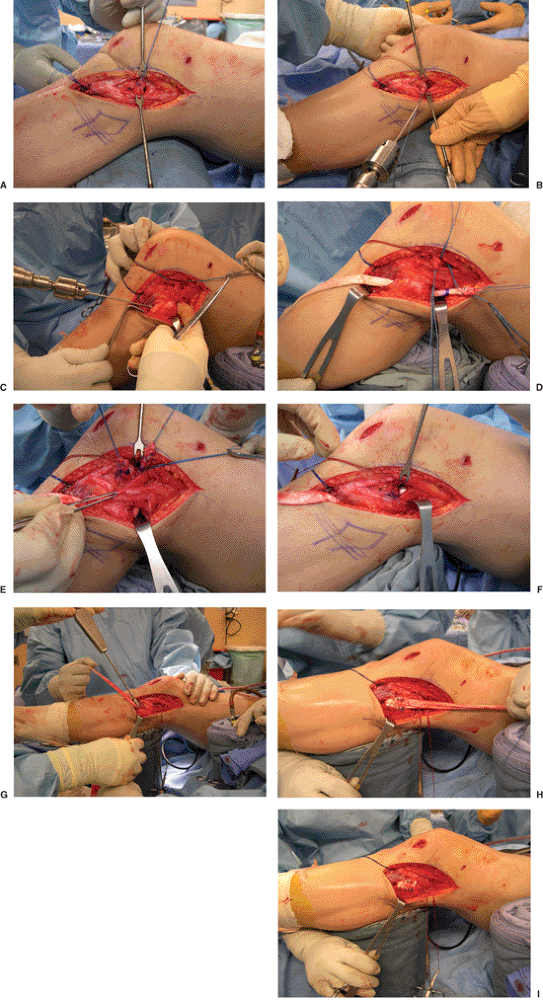
We prefer to use a tibialis anterior allograft. The lateral epicondyle
is exposed and the popliteus tendon is dissected off. A whipstitch is
used to mark the popliteus tendon. A 6-mm tunnel is drilled through the
femur at the popliteus insertion site to a depth of 25 to 30 mm. The
tunnel is dilated to 7 mm.
is dissected out and exposed with a horizontal incision just below the
biceps insertion. The PFL insertion is more proximal and medial on the
fibular head than is the LCL. The anterior portion of the fibula is
also exposed and a 3/32-inch guide wire loaded on a chuck is passed
from anterior to posterior in an attempt to match the oblique
angulation of the fibular head. The tunnel for this graft is then
drilled over the guide wire by hand with a 6-mm drill and dilated to a
diameter of 7 mm.
The
graft is passed through the tunnel from posterior to anterior with the
assistance of a Hewson suture passer. The proximal portion of the graft
is then passed underneath and medial to the LCL and inserted via Beath
pin into the previously drilled femoral tunnel. Approximately 25 mm of
the doubled tibialis allograft is placed into to the tunnel,
accompanied by approximately 10 mm of the imbricated popliteus tendon.
This graft is secured by tying the sutures over a button place on the
medial femoral cortex or tied over a post using a standard 4.5-mm AO
screw and washer. This graft is tensioned with the knee in 30 degrees
of flexion, using a bioabsorbable interference screw for fixation in
the fibular tunnel (Fig. 25.10).
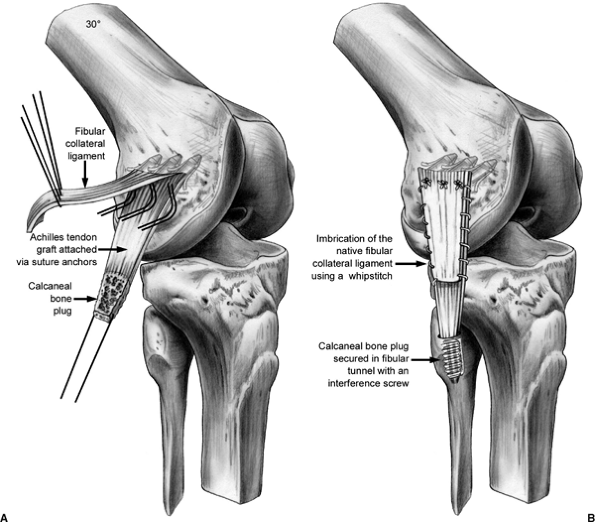 |
|
Figure 25.8. LCL reconstruction with Achilles tendon allograft.
|
 |
|
Figure 25.9. PFL reconstruction.
|
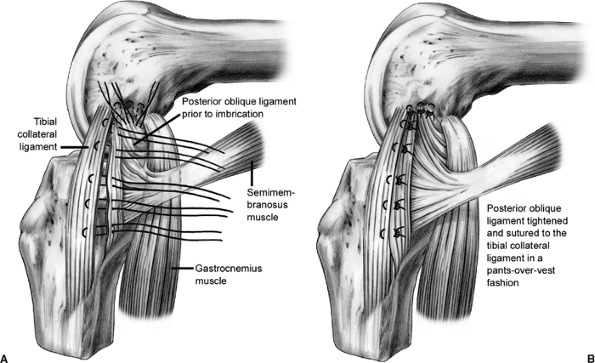
proximal in curvilinear fashion to address medial injury. We feel that
concomitant medial collateral ligament (MCL) injuries should only be
repaired for a grade III injury in which the medial side opens up in
full extension to valgus stress testing. We believe that the posterior
oblique ligament
(POL),
which is confluent with the posterior edge of the superficial MCL,
plays an integral role in medial knee stability. If repair is
indicated, a plane is established by incising longitudinally between
the POL and the superficial MCL. The medial meniscal attachments to the
POL are released back to the posteromedial corner of the knee. The
peripheral border of the meniscus is rasped to prepare a bed for the
repair of the POL. The POL is advanced anteriorly and imbricated to the
superficial MCL in pants-over-vest fashion using suture (Fig. 25.11).
The meniscus is repaired using full thickness sutures in an outside-in
manner. In the acute setting, the MCL can usually be repaired primarily
with intrasubstance nonabsorbable suture using a modified Kessler
stitch. In the chronic setting, a reconstruction may be required to
augment the repair.
 |
|
Figure 25.10. Intraoperative pictures of lateral-sided reconstruction demonstrating the following: (A) identifying, detaching, and tagging the popliteus tendon; (B) drilling of the femoral tunnel; (C) drilling of the fibular head tunnel; (D) passing the graft through the fibular head; (E) passing the graft with the popliteus tendon under the LCL; (F) pulling the graft and popliteus tendon through the femoral tunnel. (G) fixation of the graft in the fibular head after tensioning; (H) reinforcing the graft by suturing it onto itself; (I) final picture of PLC reconstruction.
|
tensioning and fixation can be accomplished in the following stepwise
manner: Our preference is first to tension and fix the PCL, then the
ACL, followed by the lateral structures, and finally the medial
structures. The PCL is fixed distally with the knee in 90 degrees of
flexion, and a bolster is placed under the tibia to support its weight
against gravity. While the assistant is performing anterior drawer, the
graft is fixed distally with an interference screw and/or a 4.5-mm AO
screw and washer via standard post fixation. The ACL graft is tensioned
and fixed distally in full extension with
an
interference screw or a screw and washer. The knee is maintained in 30
degrees of flexion for fixation of both the PLC and LCL grafts. The PLC
is reduced and fixed with an internal rotation force applied to the
tibia relative to the femur. The MCL is fixed in 30 degrees of flexion.
(Figs. 25.12,25.13,25.14).
 |
|
Figure 25.11. POL imbrication procedure.
|
a complete range of motion. An examination for stability is also
performed.
 |
|
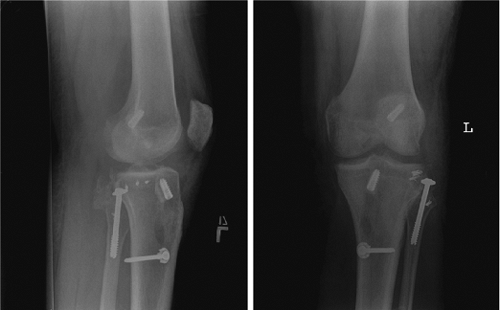
Figure 25.12. Postoperative radiographs of ACL/PCL/LCL reconstruction with PLC primary repair.
|
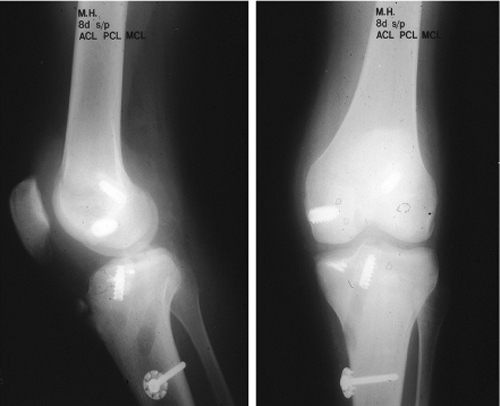 |
|
Figure 25.13. Postoperative radiographs of ACL/PCL/MCL reconstruction.
|
is performed. A standard sterile dressing is applied and a brace is
applied with the knee locked in full extension. Deep venous thrombosis
(DVT) prophylaxis is give to high-risk patients.
the first 4 weeks. The goals during the early postoperative period
include protecting the healing structures, regaining full passive
extension, and maximizing quadriceps firing. Isometric quadriceps sets
with the knee in full extension are encouraged in the immediate
postoperative period. At the 2 weeks postoperative visit, the physical
therapist will begin passive flexion, limited to 90 degrees, while
applying an anterior directed force to the proximal tibia to prevent
posterior tibial subluxation. Active flexion is prohibited in the first
6 weeks postoperatively to prevent hamstring-induced posterior tibial
translation. Crutch weight bearing is progressed from partial weight
bearing to as tolerated over the first 4 weeks. If a lateral sided
repair or reconstruction was performed, the patient remains doing
partial weight bearing until good quadriceps control is regained. At
that time, the brace is unlocked and controlled gait training is
commenced.
Quadriceps
strengthening is progressed from the isometric sets to limited arc,
open-chain, extensionexercises from 60 to 75 degrees only. Exercises
are limited to this motion arc to prevent excessive stress from being
placed on the reconstructed grafts. At 6 weeks, passive and
active-assisted range of motion and stretching exercises are begun to
regain knee flexion. The brace is discontinued after 6 weeks. At 12
weeks, open-chain hamstring exercises may be performed. Patients may
return to sedentary work in 2 to 3 weeks or heavy labor in 6 to 9
months. Patients are allowed to return to sports in 9 to 12 months.
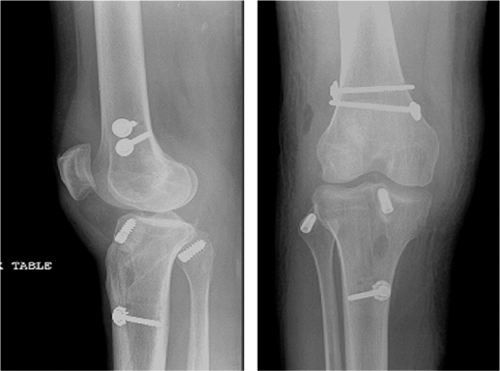 |
|
Figure 25.14. Postoperative radiographs of ACL/PCL/PLC reconstruction.
|
complications, compartment syndrome, neurovascular injury, and residual
laxity. Our surgical technique aims at minimizing these devastating
complications.
-
Wound complications: Our skin incisions
for the procedure are well planned to avoid pitfalls with exposure or
wound complications. Consequently, we prefer an arthroscopic approach
when possible, and we also prefer using allografts as opposed to
autografts. For medial and/or lateral reconstructions, we do not
recommend a midline incision because it provides limited access to the
collaterals and may be complicated by skin sloughing over the patella.
In the rare instance that both medial and lateral incisions are
required, then at least 10 cm between incisions is recommended. -
Compartment syndrome: When performing
arthroscopy for multiple ligament surgery, it is important to maintain
a low intra-articular fluid pressure to prevent extravasation of fluid.
Consequently, we do not use a pump. Extravasation into the leg and
thigh may result in a compartment syndrome. Therefore, the leg and
thigh are intermittently palpated throughout the case to assure that
the compartments are soft. If there is any question about the
vascularity or leg compartment pressures, then the arthroscopic
irrigation is stopped and the procedure is completed in a dry
arthroscopic field or with an open technique. If necessary, compartment
pressures are measured and fasciotomies performed. -
Neurovascular injury: Popliteal
neurovascular injury is a devastating complication and possible during
creation of the PCL tibial tunnel. Consequently, we use a posteromedial
portal to visualize and palpate the posterior cortex. In addition, we
use fluoroscopy during placement of the guide wire and drilling of the
tunnel, and we protect the guide wire and drill tip from posterior
penetration with a curette. During the lateral reconstruction, it is
imperative to visualize and protect the peroneal nerve, especially when
drilling the fibula. With a medial approach, the saphenous nerve should
be identified and protected throughout the case. -
Residual laxity: Laxity of the grafts
will occur if they are not tensioned and secured appropriately. The PCL
graft must be secured first to restore the step-off of the tibial
plateau. Next, the ACL should be tensioned to ensure reduction of the
tibiofemoral joint. The medial or lateral reconstruction is completed
last. Strict adherence to the postoperative protocol is critical so the
grafts are not stretched.
H, Berger DL. Blunt lower-extremity trauma and popliteal artery
injuries: revisiting the case for selective arteriography. Arch Surg 2002;137:585–589.
CG, Edson CJ. Arthroscopically assisted combined anterior and posterior
cruciate ligament reconstruction in the multiple ligament injured knee:
2- to 10-year follow-up. Arthroscopy 2002;18:703–714.
JC, Eilers AF. The role of the posterior oblique ligament in repairs of
the acute medial (collateral) ligament tears of the knee. J Bone Joint Surg Am 1973;55(5):923–940.
PP, Margheritini F, Camillieri G. One-stage arthroscopically-assisted
anterior and posterior cruciate ligament reconstruction. Arthroscopy 2001;17:700–707.
FE, Dennis JW, Veldenz HC, et al. Confirmation of the safety and
accuracy of physical examination in the evaluation of knee dislocation
for injury of the popliteal artery: a prospective study. J Trauma 2002;52:247–251.
FR, Barber-Westin SD. Reconstruction of the anterior and posterior
cruciate ligaments after knee dislocation: use of early protected
postoperative motion to decrease arthrofibrosis. Am J Sports Med 1997;25:769–778.
M, Freedman E. Allograft reconstruction of the anterior and posterior
cruciate ligaments after traumatic knee dislocation. Am J Sports Med 1995;23:580–587.
AR, Arden GP, Rainey HA. Traumatic dislocation of the knee: a report of
forty-three cases with special reference to conservative treatment. J Bone Joint Surg Br 1972;54:96–102.
DC, Becker JR, Dexter JG, et al. Reconstruction of the anterior and
posterior cruciate ligaments after knee dislocation: results using
fresh-frozen nonirradiated allografts. Am J Sports Med 1999;27:189–196.
