FRACTURES OF THE CARPAL BONES
Department of Orthopaedic Surgery, New York University School of
Medicine, Hospital for Joint Diseases; Lenox Hill Hospital, New York,
New York 10128.
percentage of traumatic injuries to the upper extremity. Frequently,
they are initially dismissed as being trivial, and medical attention is
delayed. Their seriousness is often not recognized until months or even
years later, when secondary arthritic changes appear. The diagnosis and
treatment of carpal fractures require an understanding of the anatomy
and mechanics of one of the most complex joints in the body.
the 16th century, when Versalius identified and numbered the carpal
bones (29). For the next several centuries, the
precise shape and articulation of each bone was known in detail, but
little attention was paid to its ligaments and even less to its
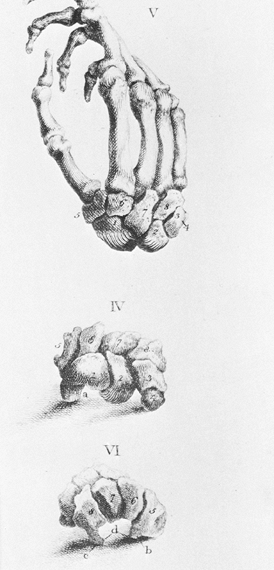
movements. Sir Charles Bell’s description of the wrist in his classic
treatise, The Hand: Its Mechanism and Vital Endowments as Evincing Design,
published in 1813, was simply: “In the human hand, bones of the wrist
are eight in number, and they are so closely connected that they form a
sort of ball which moves on the end of the radius” (Fig. 42.1).
It was not until the discovery of x-rays in 1895 that the study of
wrist mechanics began. The following year, Bryce observed that the
physiologic axis of the wrist was not fixed but shifted as the joint moved from flexion to extension (4).
These studies were later enlarged by other investigators, who
recognized the complex and synchronous movements of the carpal bones,
movements that impart to the wrist both fluidity of motion and strength
(60,61).
 |
|
Figure 42.1. Bones of the hand and wrist, from a 19th-century anatomy book by Charles Bell (7). The carpal bones at that time were referred to by numbers.
|
The medial column was for rotation and included the triquetrum and
pisiform, and the lateral column was for mobility and included the
scaphoid, trapezium, and trapezoid. In 1943, Gilford described wrist
mechanics differently (37). He likened the
wrist to a triple-link system with the radius, lunate, and capitate as
the central link. The mechanical advantage of the system is that its
two main joints, the radiolunate and lunate–capitate, move only half
the excursion of the entire joint. The disadvantage of the system is
instability of the central, intercalated mobile segment (i.e., lunate),
which is dependent on its anatomic configurations and ligament
attachments for stability. More than 50 years after Navarro published
his initial account, Taleisnik modified his concept of the columnar
carpus by including the entire distal carpal row with the lunate in the
central column (92). Taleisnik also limited the
medial (rotational) and lateral (mobile) columns to one bone each, the
triquetrum and scaphoid respectively. In both the columnar and
triple-link concepts of wrist mechanics, the scaphoid is the key bone
for carpal stability. Although the scaphoid is anatomically located
within the proximal carpal row, it functionally bridges both carpal
rows.
physical examination, including the neurovascular status of the hand.
Determining precise areas of tenderness aids in localizing the site of
injury. Conventional radiographs are always necessary, and generally
four views are obtained: posteroanterior (PA), anteroposterior (AP),
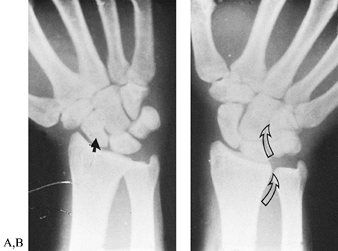
lateral, and oblique (Fig. 42.2). The PA view
in ulnar deviation visualizes the scaphoid, whereas the AP view (palm
up or supinated view), usually taken with the fingers clenched and
wrist ulnarly deviated, is useful for visualizing rotatory subluxation
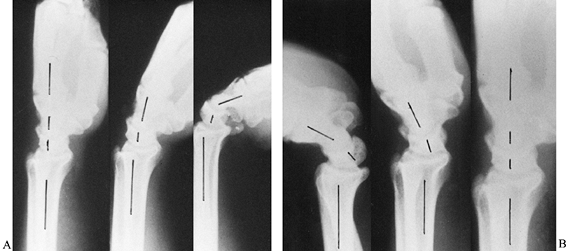
of the scaphoid. The lateral radiograph should be a true lateral view
with the wrist in neutral position in order to evaluate carpal bone
alignment (Fig. 42.3). Oblique views can be
taken with the hand slightly pronated (posterior oblique) or slightly
supinated (anterior oblique). The posterior-oblique view visualizes the
carpal bones on the radial side of the wrist, particularly the
scaphoid, and the anterior-oblique view visualizes the pisiform and, to
a lesser extent, the hamate. Additional imaging techniques are
sometimes required, including: carpal tunnel views; tomography, either
computer assisted (CT) or polyaxial; arthrography; and magnetic
resonance imaging (MRI). The CT is the most frequently used special
imaging study for detecting carpal fractures and is obtained in at
least two planes, using 2-mm slices. It has generally replaced
polyaxial tomography (i.e., trispiral tomography) because of its wider
availability and superior images. In addition to detecting occult
fractures, CT is
useful
for assessing fracture healing and healing of bone grafts used for the
treatment of nonunions. It also permits visualization of precise bony
detail that aids in the evaluation of other conditions such as
intraosseous tumors or cysts.
 |
|
Figure 42.2. Posteroanterior views of a normal wrist in radial and ulnar deviation. A: In radial deviation, the scaphoid is palmar flexed, and its shortened appearance is sometimes misinterpreted as a fracture (closed arrow). B:
The outline of the scaphoid is best visualized with the wrist in ulnar deviation. This wrist position also demonstrates the relationships between the forearm and carpal bones. In ulnar deviation, the radioulnar, lunate-triquetral, and capitate-hamate joints (open arrows) line up to form a sinusoidal curve. |
 |
|
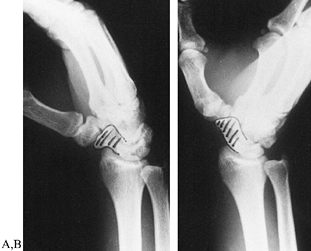
Figure 42.3. A,B:
Lateral radiographs of a normal wrist show the alignment of the radius, lunate, capitate, and metacarpals in neutral position and the movements of the radiocarpal and midcarpal joints with the wrist in slight and complete palmar flexion and dorsiflexion. Cineradiographic studies have shown that during the initial 30° of palmar flexion or dorsiflexion, motions at both radiocarpal and midcarpal joints are approximately equal. Further movements in either direction occur primarily at the midcarpal joint. The longitudinal axis of the scaphoid is at an angle of 45° to 60° to the longitudinal axis of the lunate with the wrist in neutral position. |
fractures. They usually occur in young adult men following falls on
their outstretched palms. Experimental studies have shown that the
force must be applied to the radial side of the palm, with the wrist
extended a minimum of 95° (102). In that
position, the scaphoid is the only carpal bone in contact with the
radius. The proximal part of the scaphoid assumes a wedge-shaped
configuration between the radius and capitate, where it is supported by
the radial collateral and radiocapitate ligaments. The distal pole of
the scaphoid, however, is unsupported and capsular structures in the
area are lax. It is the distal pole that receives most of the applied
force and the bone fractures at its most vulnerable area, its waist.
over the dorsal surface of the bone in the anatomic snuff box or over
its tubercle on the palmar surface. Confirm
the
diagnosis with radiographs. The profile of the bone is best seen on PA
and posterior-oblique views with the wrist in ulnar deviation (Fig. 42.4).
Although radiographs taken immediately after the injury may be
negative, the scaphoid should still be considered fractured until
proven otherwise. Immobilize the wrist in a thumb spica splint or cast
and repeat radiographs in 1 to 2 weeks. If there is a fracture, it
should then be evident by the appearance of bone resorption at the
fracture site. Occasionally, bone resorption does not appear until 3
weeks after the fracture, and even then radiographs may remain
inconclusive. In these cases, radionuclide imaging with technetium-99m (99mTc) can be helpful (32).
Although bone scans are highly sensitive and are generally positive
within 24 h of a fracture, they are nonspecific. Therefore, although a
fracture can be ruled out with a negative scan, a positive scan
requires more specific imaging studies. Vibratory testing, using
audible “intrasound” frequencies between 20 and 20,000 Hz (infrasound
less than 20 Hz and ultrasound greater than 20,000 Hz are inaudible),
has been shown to be effective in diagnosing occult scaphoid fractures (8).
The test is considered positive when pain is sufficient to cause an
immediate “positive retraction response.” The definitive test is a CT
scan.
 |
|
Figure 42.4. Posterior oblique radiographs of a normal wrist in radial (A) and ulnar (B) deviation. The scaphoid (shaded) is best visualized with the wrist in ulnar deviation.
|
variety of methods. The two most common are the relationship of the
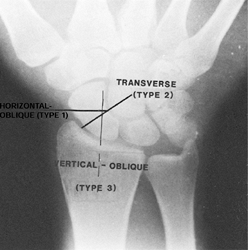
fracture to the longitudinal axis of the scaphoid (Russe’s
classification) and the site of fracture. Russe’s classification
comprises three types of fractures (Fig. 42.5) (80). Type I is a transverse-oblique
fracture that is horizontal to the wrist joint and oblique to the
longitudinal axis of the scaphoid. These fractures comprise
approximately 35% of scaphoid fractures (90). Type II is a transverse
fracture at right angles to the longitudinal axis of the scaphoid. It
is the most common type of scaphoid fracture (60%). Type III is a vertical-oblique
fracture that is vertical to the wrist joint and oblique to the
scaphoid. Although vertical-oblique fractures are the rarest and occur
in only 5% of scaphoid fractures, they are the most problematic with
respect to healing because they are subject to high shear forces and
tend to be unstable.
 |
|
Figure 42.5. Classification of scaphoid fractures according to Russe.
|
thirds. Most fractures involve the middle third of the bone. They
usually heal if they are not displaced, and there is no intercarpal
instability. Fractures in the proximal third, sometimes referred to as
the proximal pole of the scaphoid, have the highest incidence of
nonunion and avascular necrosis because of the pattern of intraosseous
circulation. The blood supply to the scaphoid enters dorsally and
distally and traverses the bone in a proximal direction (36).
Fractures in the distal third of the scaphoid are the rarest and tend
to heal promptly because of the excellent blood supply in the area.
Distal-third fractures include intraarticular fractures and fractures
of the tubercle. Intraarticular fractures can cause articular
incongruity when they are vertically oriented. They require operative
reduction and internal fixation to avoid later arthritis at the
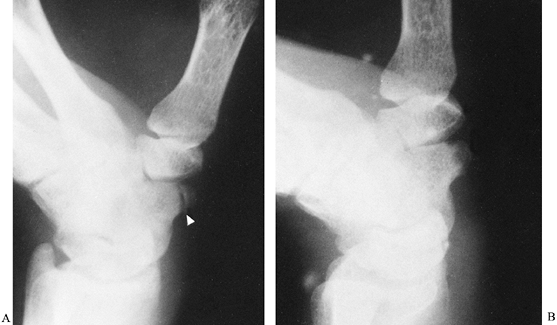
scaphotrapezial joint. Fractures of the scaphoid tubercle usually
result from direct trauma (Fig. 42.6). Treatment is directed primarily at relieving
pain; this can be achieved by wearing a wrist splint for several weeks.
There are few consequences of a tubercle fracture failing to heal
because the bony prominence is extraarticular. Persistent pain is
uncommon, but if it does occur the tubercle can be excised without
compromising wrist function.
 |
|
Figure 42.6. A: Fracture of a scaphoid tubercle (arrowhead). B: The fracture healed after 6 weeks of immobilization.
|
when diagnosed within 3 to 4 weeks of the injury. Healing can be
expected in more than 95% of cases with thumb spica cast
immobilization, provided the fracture is nondisplaced. When diagnosis
is delayed more than 4 weeks, the prognosis is much worse, and failure
to heal ranges from 40% to as high as 88% (49). The time period for what constitutes delayed union or nonunion
is controversial because both terms have been applied to scaphoid
fractures that have not healed after 4 to 6 months of immobilization (79,91).
This is an arbitrary time period because it is impossible to state with
certainty when delayed union begins. Although it is not unreasonable to
label a scaphoid fracture that has not united in 4 to 6 months a
“delayed union,” it is often premature to refer to a similar problem
within that same time period as a “nonunion.” Unlike nonunions, delayed
unions still have the capacity to heal with continued immobilization,
which can sometimes take longer than 6 months. A nonunion is
essentially a radiographic diagnosis that comprises specific criteria
that are usually more obvious when the nonunion is hypertrophic rather
than atrophic. Hypertrophic nonunions are characterized by sclerosis at
the fracture site, which gives the appearance of a pseudarthrosis.
Changes in atrophic nonunions tend to be less obvious; the fragments
are osteoporotic, and the fracture margins irregular and cystic. The
radiographic changes in both types of nonunions are most clearly seen
with CT imaging.
who observed that when a scaphoid fracture is displaced, the proximal
fragment flexes together with the adjacent lunate. This pattern results
in a zig-zag deformity at the midcarpal joint, which has been likened
to the bellows of a concertina and referred to as a “concertina
collapse deformity” (9). Reducing the proximal
fracture fragment restores normal tension to the palmar ligaments and
corrects the abnormal rotation of the lunate (66). Carpal collapse associated with a displaced scaphoid fracture is generally described as dorsal intercalated segment instability or DISI (53).
Unacceptable fracture displacement has been defined as 1 mm or more
step-off on PA and/or oblique radiographs, greater than 15° angulation
between lunate and capitate, and scapholunate angulation greater than
45° (8). In summary, the factors that have a negative impact on healing are obliquity of the fracture line, a fracture in the proximal
pole of the scaphoid, displacement and/or angulation of the fracture, and a delay in diagnosis.
well-molded thumb spica cast extending just distal to the
interphalangeal joint, permitting only slight flexion of that joint.
Because tension of the radial collateral ligament of the wrist can
cause displacement at the fracture site, immobilize the wrist in slight
flexion and radial deviation. Avoid forearm rotation by applying a
Muenster cast, which permits elbow flexion and extension. After 8
weeks, use a below-elbow thumb spica cast until there is radiographic
evidence of healing. It is critically important to document that
radiographic healing is complete before immobilization is discontinued.
particularly when conventional radiographs appear to show a healed
fracture but tenderness persists at the fracture site. If healing is
incomplete, continue cast immobilization and obtain new radiographs in
6 to 8 weeks.
 |
|
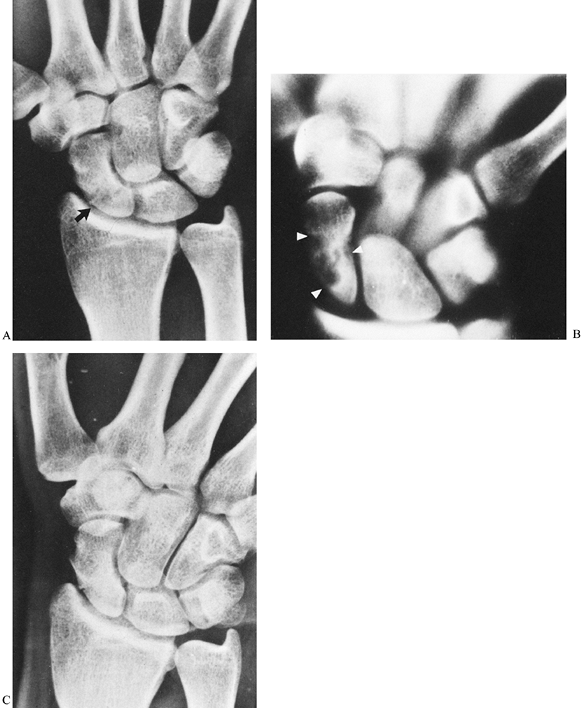
Figure 42.7. A:
Trispiral tomograph after 3 months of cast immobilization for a fracture in the proximal one-third of the scaphoid. Although there was no tenderness at the fracture site, healing was questionable because the fracture line was still visible (arrow). B: Tomographs confirmed the suspicion that the fracture had not healed, and a large area of radiolucency (arrows) was visualized that was not apparent on the conventional radiographs. C: After an additional 2 months of cast immobilization, the fracture healed. |
may occasionally be indicated in patients (e.g., surgeons, dentists)
who cannot work with their wrist in a cast and would face serious
financial hardship if disabled for months. Bone screws are now
available that provide stable fixation and compression of scaphoid
fractures such as a cannulated AO/ASIF screw (Synthes, Paoli, PA) or
Acutrak headless screw (Acumed, Beaverton, OR). Intraoperative
radiographic imaging is essential to ensure correct placement of the
screw (Fig. 42.8).
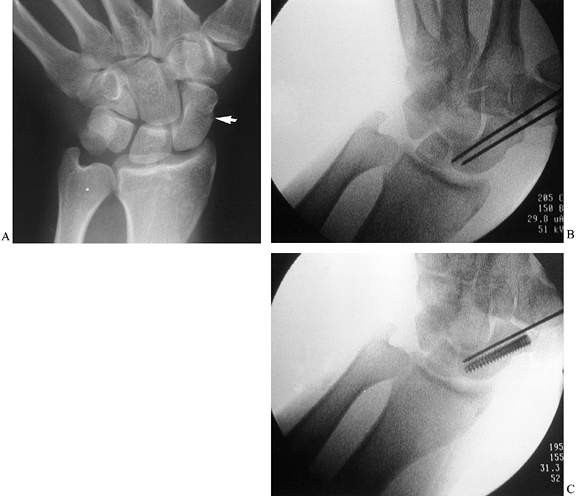 |
|
Figure 42.8. A nondisplaced scaphoid fracture (arrow) in a 40-year-old physician. The fracture healed in 2 months following insertion of an Acutrak headless, cannulated screw.
|
-
Under direct radiographic imaging, drill a pin down the axis of the bone (Fig. 42.8B, arrow).
-
Insert a second pin parallel to the first to prevent rotation and possible displacement of the fracture.
-
Drill an Acutrak cannulated screw over the first wire; then, remove the second wire (Fig. 42.8C).
for a week or two until there is soft-tissue healing and then convert
to a well-molded thermoplastic thumb spica splint. The patient can then
return to light work activities that do not require heavy lifting,
pushing, or pulling. For surgeons, the splint can be removed while
operating.
have not healed after months of immobilization are different from
fractures that were unrecognized until months after the injury. In the
first situation, union is delayed but can still occur with continued
immobilization, provided the fracture is stable and there are no
radiographic signs of nonunion (i.e., sclerosis and/or cyst formation).
Radiographic evidence of avascular necrosis is not an indication that
the fracture will not heal, although it may take longer to heal. For
scaphoid fractures unrecognized until months after the injury, the
likelihood for healing with cast immobilization is poor.
debilitated individuals. It is one of a few orthopaedic problems in
which the indication for surgery depends on the radiographic appearance
of the bone and not on the severity of patient’s symptoms. Surgery is
usually required even in patients who are asymptomatic and have
excellent wrist mobility. Prospective studies have shown that without
surgery, scaphoid nonunions are likely to lead to traumatic arthritis
accompanied by pain, loss of wrist mobility, and weakness (54,57,78).
recommended for scaphoid nonunions, including drilling the bone,
excising the proximal fragment or even both fragments, excising the
proximal carpal row, intercarpal and total wrist arthrodeses,
prosthetic replacement of the scaphoid, radial styloidectomy,
interposition of soft tissue into the nonunion site, and autogenous
bone grafting (9,83).
Drilling the bone is only of historic interest and has little relevance
to contemporary hand surgery. Excising the proximal fracture fragment
is a useful procedure provided the fragment is small, not exceeding 25%
of the length of the bone (Fig. 42.9). The
fragment can sometimes be removed arthroscopically. Avoid excising
larger fragments because it can lead to abnormal shift of the other
carpal bones, particularly the capitate. A coiled tendon graft can be
inserted into the void as a plug, although it is not essential.
Proximal row carpectomies and arthrodeses, whether they are partial or
complete, are salvage procedures and are indicated when secondary
arthritic changes have developed. Replacing the scaphoid with a
silicone prosthesis is another salvage procedure that was popular until
the mid-1980s. Since then, the procedure has been abandoned because of
its high complication rate, including dislocation, breakage, and, most
important, silicone synovitis (5,85).
Titanium carpal implants are available, but they have not gained wide
acceptance because they are inherently unstable and frequently cause
bone erosion. Interposing a soft tissue flap between the fracture
fragments was a procedure first recommended by Bentzon in 1940. It is
still recommended by some when bone grafting is unsuccessful.
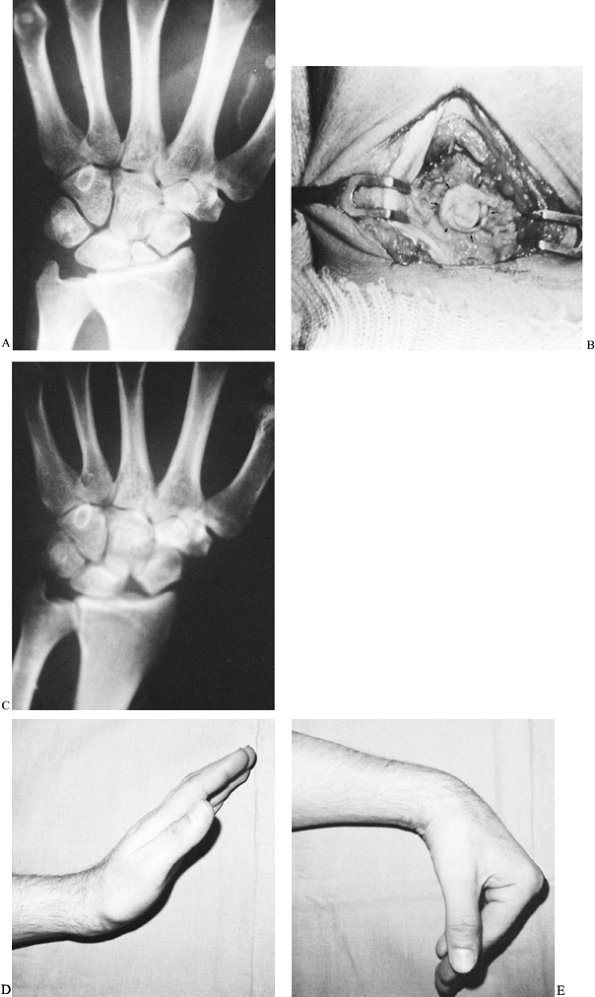 |
|
Figure 42.9. A:
Radiograph of a scaphoid nonunion in a 38-year-old accountant. The likelihood of achieving healing is minimal with a bone graft when there is a small proximal fracture fragment whose sclerotic appearance indicates avascular necrosis. B: The proximal fragment was excised, and a coiled piece of palmaris longus tendon (arrows) was inserted into the void. C: A radiograph 2 years later showed no secondary shifting of any carpal bone. D,E: The patient regained satisfactory pain-free wrist mobility. |
-
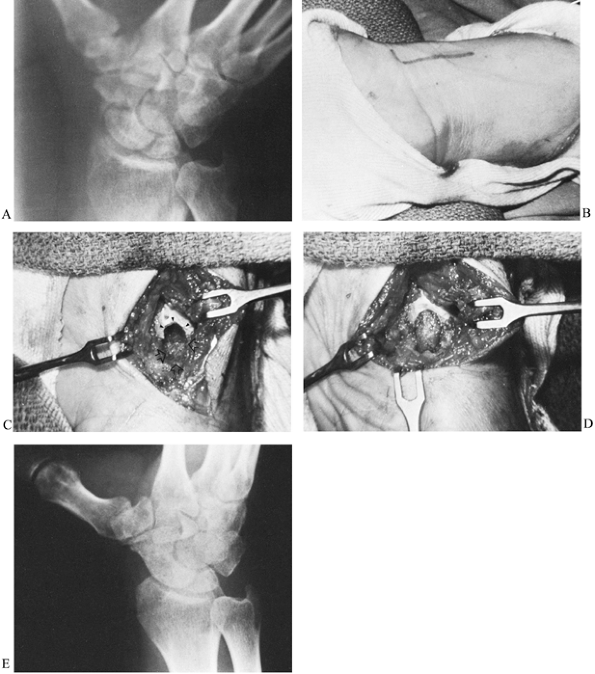
Make a 3.0- to 4.0-cm longitudinal incision along the radial border of the flexor carpi radialis (FCR) tendon (Fig. 42.10). Curving the distal portion of the incision into the wrist flexion crease facilitates exposure.
![]() Figure 42.10. A: Radiograph of a nonunion of the scaphoid. B: At 3 months postoperatively, the fracture had healed and there was radiographic evidence of bone revascularization. C: A deep trough across the fracture site (arrows). D: Trough filled with an autogenous corticocancellous iliac bone graft. E: Radiograph taken 4 months after surgery shows complete consolidation of the graft.
Figure 42.10. A: Radiograph of a nonunion of the scaphoid. B: At 3 months postoperatively, the fracture had healed and there was radiographic evidence of bone revascularization. C: A deep trough across the fracture site (arrows). D: Trough filled with an autogenous corticocancellous iliac bone graft. E: Radiograph taken 4 months after surgery shows complete consolidation of the graft. -
Dissect the interval between the FCR and radial artery, and incise the joint capsule longitudinally.
-
Identify the nonunion. The radial styloid serves as a guide to its location.
-
Using a power burr, make a deep trough across the nonunion site (Fig. 42.10C, arrows).
Curve the trough slightly to follow the contour of the scaphoid.
Undercut its periphery to enhance stability of the bone graft. -
Harvest a corticocancellous graft from
the outer surface of the ilium, just below the crest. Some prefer to
harvest a graft from the distal radius; however, cancellous bone at
that site is not nearly as dense as in the ilium. Shape the graft using
a small bone cutter to be slightly larger than the trough so that it
will fit snugly. -
Insert the graft with its thin cortical
surface facing outward to be the covering of the trough. Only
cancellous bone is in contact with the nonunion site (Fig. 42.10D).
Fill any remaining defects with cancellous chips. With stable
nonunions, the graft can be totally cancellous. Internal fixation with
Kirschner wires is necessary only when there is movement at the
nonunion site after the graft is inserted.
-
Green (38)
recommends that power instruments not be used when excavating the
trough because of the risk of bone overheating. He uses two
corticocancellous struts inserted into the trough with their cancellous
surfaces together and cortical surfaces facing outward against the
inner walls of the nonunion site to provide better stability. -
Green also reported that bone grafting is
contraindicated in the presence of avascular necrosis of the proximal
fragment, which is evident by the absence of punctate bleeding points
in the bone. -
His recommendations differ from the
experiences of most surgeons. A power burr facilitates preparation of
the trough, which is the most important technical part of the
operation, and the risk of bone damage is avoided by using irrigation. -
Cancellous bone rather than cortical bone should be positioned against the nonunion site.
a double-threaded compression screw for the treatment of scaphoid
nonunion. The procedure is technically demanding, and its benefits
remain unproven. Newer and more effective screws are now available that
are cannulated and provide greater compression (e.g., AO/ASIF screw,
Acutrak tapered headless screw) (81).
problem because it is frequently associated with subluxation of the
lunate. The lunate, which is attached to the scaphoid by a strong
interosseous ligament, follows the malpositioned proximal fragment,
resulting in a “concertina” deformity or DISI deformity at the
midcarpal joint. The nonunion site is flexed with apex dorsal
angulation, and the bone has a “humpback” deformity. The degree of
deformity may not be obvious on conventional radiographs, but it is
clearly demonstrated on lateral CT.
-
Measure the intrascaphoid
angle that is formed by the intersection of lines drawn perpendicular
to the proximal and distal articular surfaces of the scaphoid. The
normal angle is 30° to 40°. An intrascaphoid angle greater than 45°
generally should be corrected (5). -
Use a palmar operative approach. When
lunate tilt is severe, a dorsal operative approach is preferable
because it provides better visualization. Care must be taken with a
dorsal approach to avoid damage to the radial artery and its dorsal
branches to the scaphoid. -
Correct lunate alignment by flexing and
ulnar deviating the wrist, and then stabilize the lunate with one or
two Kirschner wires. -
Confirm normal radiocarpal and midcarpal alignment using intraoperative radiographic imaging.
graft provided the criteria present when the first operation was
performed still exist. The scaphoid should not be fragmented, and there
should be no significant arthritic changes. Pulsed electromagnetic
fields (PEMP) should also be considered (2,11).
Although double-blind studies have not been performed to prove its
efficacy, my experience is that it improves the chances for healing.
Another surgical option is a vascularized bone graft. Several donor
areas have been suggested, and probably the most effective is one
suggested by Zaidemberg et al. (105). They
recommended a graft harvested from the dorsoradial aspect of the distal
radius, a site supplied by an ascending branch of the radial artery.
Vascularized bone grafts have also been recommended for avascular
necrosis of the scaphoid. The advantages of these procedures over
conventional bone grafting have yet to be established by clinical
studies.
because of the risk that a corrective osteotomy may fail to unite and
lead to an even greater problem, a nonunion. Surgery is not a difficult
decision for the patient who has a significant disability from wrist
pain, tenderness, and diminished grip strength. The decision is more
difficult when there are a paucity of symptoms. However, malalignment
of the midcarpal joint results in altered wrist kinematics that are
likely to lead eventually to arthritic changes. Therefore, surgery is
generally recommended for young patients. Careful preoperative planning
is critically important and requires CT imaging.
-
Use a dorsal operative approach.
-
If the position of the lunate can not be
corrected by wrist flexion and ulnar deviation, drill a Kirschner wire
into the bone to serve as a joystick.
-
A radial styloidectomy should not be
routinely performed as an adjunct to bone grafting. It is indicated for
those rare cases of symptomatic arthritis confined to the area between
the styloid process and the scaphoid.
-
Use interoperative imaging to insure that carpal alignment has been restored.
-
Osteotomize the scaphoid using a thin osteotome or a power saw.
-
Insert a bone graft similar to the method used for grafting a malpositioned nonunion.
condition commonly referred to as “Preiser disease.” Actually, this is
a misnomer because the patients Preiser described in his paper
published in 1910 all had sustained prior injuries (25).
When there are no apparent causes, it is more appropriate to refer to
the condition as “idiopathic avascular necrosis of the scaphoid.” It
may be similar to avascular necrosis of the lunate (Kienböck’s disease)
in that variations in the blood supply to the bone may predispose
certain individuals to the condition. Trauma may be the main factor in
disrupting the blood supply to the bone, but it may be so trivial that
patients are unable to recall the episode(s) (74).
Treatment depends on the condition of the scaphoid. In the absence of
arthritic changes, a vascularized bone graft should be considered. When
disease is chronic and pain is disabling, a salvage procedure would be
required such as proximal row carpectomy, scaphoid excision combined
with midcarpal arthrodesis, or total wrist arthrodesis.
Before ossification or in the early stages of ossification, scaphoid
fractures are exceedingly rare because the bone is almost entirely
cartilaginous, which cushions the effects of trauma. Even in the later
stages of ossification, a considerable portion of the bone remains
cartilaginous, and fractures are less likely to occur than when the
bone is fully mature. Scaphoid fractures do occur in children, however;
these fractures generally occur after the age of 9, unless there is
precocious ossification. Most fractures involve the distal third of the
bone or its tubercle (39). The incidence of fractures through the middle third of the bone is only 10% as compared to a 70% incidence in adults (97).
Scaphoid fractures in children usually heal within 8 weeks. Nonunions
are rare, but when they occur bone grafting is necessary (Fig. 42.11).
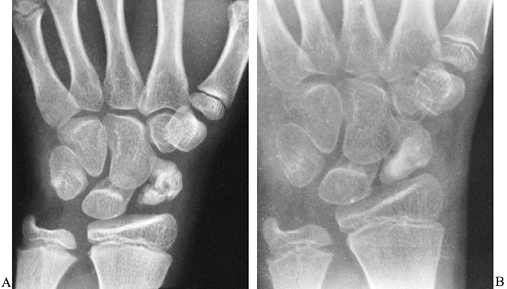 |
|
Figure 42.11. A:
Radiograph of the wrist of an 11-year-old child shows a nonunion of the scaphoid. There was a history of wrist injury more than 1 year earlier for which medical treatment was never obtained. At surgery, during preparation of the trough for the bone graft, there was minimal bleeding from either fracture fragment, consistent with the radiographic appearance of avascular necrosis. B: Healing was almost complete 3 months after surgery. |
can pose a diagnostic dilemma, especially when there is separation of
the fragments. Radiographs may show an
actual fracture or what some believe is a developmental variation, a bipartite scaphoid arising from two ossification centers (101).
Absence of a history of trauma, equal size and density of both bones
with a clear space between them, and contiguous surfaces that are
smooth have been cited as indications that a bipartite scaphoid is a
developmental condition (69). These
radiographic findings can also be associated with a fracture, however.
Although some bipartite scaphoids can be developmental in origin, most
result from trauma (56,69).
More important than their etiology is their clinical course. Because
they frequently are associated with later arthritis, treatment is
warranted (82,101).
Electrical stimulation has been suggested as a method of treatment, and
little is lost by trying it. If it is not effective after 4 to 6
months, however, consider bone grafting.
The incidence varies widely because most are chip fractures from the
dorsal cortex of the bone that often go unnoticed. The mechanism of
injury for these fractures is somewhat controversial. Initially, they
were considered avulsion injuries caused by sudden wrist flexion;
however, they almost always result from direct impact from the styloid
process of the ulna (33,51).
When the wrist is in maximum extension and ulnar deviation, the styloid
process, which is often prominent in patients who sustain these
injuries, functions as a chisel as it strikes the triquetrum and shears
off a fragment from its dorsal aspect. Lateral radiographs frequently
show the bone fragment to be at the level of the midcarpal joint, and
it is sometimes misdiagnosed as a fracture fragment off the lunate. A
posterior-oblique radiograph will usually demonstrate the defect in the
triquetrum (2). To treat, immobilize the wrist
for approximately 6 weeks, or until pain and tenderness have
disappeared. Bone fragments that are nondisplaced or minimally
displaced usually heal within several months. Displaced fragments that
fail to unite are rarely symptomatic enough to warrant excision.
and are either linear or comminuted. Comminuted fractures are commonly
associated with dorsal chip fractures and probably occur by the same
mechanism but are more severe. Treat with immobilization for 6 to 8
weeks. Although nonunions do occur, avascular necrosis has not been
reported (3,23).
These fractures are significant injuries when displaced because they
affect the important trapeziometacarpal joint of the thumb. If not
reduced, they may lead to pain, limitation of the thumb mobility, and
weakness. Trapezial fractures fall into two categories: fractures
involving the palmar ridge of the bone, and vertical fractures through
the body of the bone.
fractures and result from falls on the outstretched palm. The ridge
fractures either by direct contact or indirectly. An indirect fracture
is an avulsion injury caused by a sudden tension force applied to the
transverse carpal ligament as the thenar and hypothenar eminences
diverge. Trapezial ridge fractures are subdivided into type I
fractures, located at the base of the ridge, and type II fractures,
located at the tip of the ridge (71). Both
types of fractures are associated with local tenderness. Pain with
resisted wrist flexion is common because of the close proximity of the
FCR tendon to the fracture site.
They probably result from sudden hyperextension–abduction of the thumb
that forces the wrist into a position of maximum radial deviation. The
trapezium is wedged between the first metacarpal and styloid process of
the radius, and the styloid, functioning as an anvil, fractures the
trapezium (30,46). The
fracture is usually located in the middle of the bone. The lateral
fragment remains tethered to the first metacarpal and is often
displaced radially and proximally by the pull of the abductor pollicis
longus, similar to the mechanism that contributes to a displaced
Bennett’s fracture. Horizontal fractures through the trapezium are rare
(45).
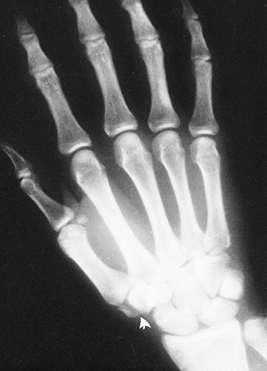 |
|
Figure 42.12. Radiograph shows a vertical fracture through the body of the trapezium (arrow).
|
frequently overlooked because of inadequate radiographs.
Posteroanterior and lateral views fail to show the entire body of the
bone: the PA view because of superimposition by the trapezoid and base
of the second metacarpal, and the lateral view because of
superimposition by the hook of the hamate. In order to visualize the
entire body of the trapezium, an oblique radiographic view is
necessary. One such view is the Bett’s view, which is obtained by
placing the ulnar border of the hand on the cassette and directing the
x-ray beam at the scaphoid–trapezium–trapezoid joints with the thumb
abducted and extended (93). Visualizing the palmar ridge requires a carpal tunnel view.
splint for 6 to 8 weeks. Type I fractures through the base of the ridge
tend to heal faster than type II fractures at the tip of the ridge.
Occasionally, a type II fracture fails to unite and causes persistent
pain and tenderness. Excision of the bony fragment is warranted in such
cases. Treatment for nondisplaced fractures through the body is similar
to that for type I and type II fractures of the palmar ridge. However,
displaced fractures resulting in joint incongruity require operative
reduction and internal fixation with Kirschner wires or a screw.
They occur as isolated injuries or in conjunction with other injuries,
particularly fractures of the scaphoid. The combination of a scaphoid
and capitate fracture was first reported by Fenton, who named the
injury “naviculocapitate syndrome” (26). This
is a complex injury that usually occurs following a fall on the
outstretched hand with the wrist extended. Fenton believed that the
scaphoid, buttressed medially by the capitate, was fractured by the
styloid process of the radius, which functioned as a chisel. When the
force was sufficiently violent, the capitate also fractured. The
scaphoid can also fracture by striking the dorsal rim of the radius.
When this occurs, the lunate extends, and the capitate
migrates
even further dorsally and fractures as it impinges against the dorsal
rim of the radius or against the dorsal rim of the lunate. Similar
fractures have been reported following a blow to the dorsum of the
flexed wrist, which causes the capitate to strike the volar lip of the
radius (98).
Regardless of mechanism of injury, the capitate usually fractures at
its neck, and the proximal fragment rotates through an arc of 180° (89).
palmar aspect of the bone. Intraosseous circulation then proceeds in a
distal-to-proximal direction, similar to the scaphoid (40).
Therefore, a fracture through the neck of the capitate jeopardizes the
blood supply to the proximal portion of the bone and can lead to
avascular necrosis. Displaced capitate fractures require operative
reduction and internal fixation (59). Nonunions, with or without avascular necrosis of the proximal fragment, require an inlay bone graft (31,62).
-
Make a transverse incision over the
dorsal aspect of the wrist. Curve the radial end of the incision
distally for 1 to 2 cm. If additional exposure is required, curve the
ulnar end of the incision proximally for the same distance. Mobilize
the skin flaps taking care to protect the sensory branches of the
radial nerve and the dorsal sensory branches of the ulnar nerve. -
Incise the extensor retinaculum
longitudinally over the fourth tendon compartment and reflect it
radially, exposing the second and third compartments. Retract the
tendons in the fourth compartment ulnarly, and the tendons in the
second and third compartments radially. -
Incise the underlying joint capsule
transversely and reflect it proximally and distally. This surgical
approach permits excellent visualization of the underlying carpal bones
and is useful for a variety of other operations, including intercarpal
arthrodeses. -
Make a deep trough across the nonunion site and pack it with a corticocancellous bone from the ilium.
-
Close the joint capsule and extensor
retinaculum. Immobilize the wrist in slight flexion to minimize the
capsulodesis effect that follows any dorsal surgical approach to the
wrist joint. Initially, apply volar and dorsal plaster splints; when
soft tissue healing is complete, usually within 2 weeks, replace the
splints with a circular cast or a well-molded plastic splint.
of cartilage (approximately 80%) of any carpal bone. Periosteum is
confined to two small areas on the volar and dorsal surfaces of the
bone through which nutrient vessels pass (95).
Injection studies have shown that in more than 90% of specimens, the
vessels form three distinct patterns of intraosseous circulation that
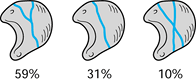
resemble the letters Y, I, and X (Fig. 42.13) (35).
The Y pattern is the most common (59%), followed by the I pattern (31%)
and the X pattern (10%). Fewer than 10% of specimens had only volar
nutrient vessels.
 |
|
Figure 42.13. The intraosseous vascular patterns in lunates.
|
This low figure may reflect a failure to diagnose many lunate
fractures. Fractures in bones that have a high proportion of cartilage
and cancellous bone, such as the lunate, often go unrecognized because
cancellous bone has a higher pain threshold than periosteum (12). Acute lunate fractures have been classified into five types (Fig. 42.14) (95):
 |
|
Figure 42.14. Classification of lunate fractures. Type I, fracture of the volar pole; type II, chip fracture away from the nutrient vessels; type III, fracture of the dorsal pole; type IV, sagittal fracture through the bone; type V, transverse fracture through the waist of the bone
|
-
I. Volar pole fractures at the entrance of the nutrient vessel.
-
II. Chip fractures not in areas of nutrient vessels.
-
III. Fractures of the dorsal pole at the entrance of the nutrient vessel.
-
IV. Sagittal fractures through the bone.
-
V. Transverse fractures through the waist of the bone.
a type IV fracture proximal to any of the three vascular patterns could
theoretically deprive the proximal portion of the bone of its blood
supply (48). Compromise to the dorsal
circulation could also occur following a type V fracture in a lunate
having only a volar nutrient vessel.
resulting from avascular necrosis remains valid to the present day, but
the cause of the condition and the most effective treatment remain
unresolved (48). In 1928, Hulten observed a
correlation between Kienböck’s disease and short ulnas and introduced
the term “ulna minus variance” (43). The role
that this anatomic variance plays in this condition remains unclear
because its incidence varies in different races. Compared to the 87%
incidence in Hulten’s series, the average incidence in Japan is only
22% (94). In the United States, ulna variance is normally more positive in black than white Americans (+0.70 mm vs. +0.27 mm) (34).
Accurate measurements are important because forearm rotation and grip
affect ulna variance. Radiographs must be carried out with the wrist in
neutral position, the forearm in neutral rotation (elbow flexed 90° and
shoulder abducted 90°), and the fingers extended. Although ulna minus
variance is not the primary etiologic factor in Kienböck’s disease, it
may increase the vulnerability of a lunate subjected to repetitive
compressive forces. Kienböck’s disease may therefore result from
unrecognized and untreated minor fractures that disrupt the blood
supply to the bone.
been general agreement about the radiographic appearance of the lunate
in the later stages of the disease. For more that 30 years, however,
opinions differed concerning the early radiographic appearance of the
bone and the timing and sequence of subsequent changes. In 1947, Stahl
attempted to bring order to a controversial subject by classifying
changes he observed in wrist radiographs in a large series of patients
with the condition (87). His classification system comprised five groups:
-
Group I. A radiodense line that
represented an acute compressive fracture of the lunate. This group of
patients was the smallest of his five groups, comprising only 2% of the
total number of cases. -
Group II. A line of rarefaction secondary
to resorption at the fracture site. Stahl thought this occurred about 1
month after the original injury. The number of patients in this group
was also small (5%). -
Group III. Sclerotic changes in close
proximity to the fracture line or in the proximal portion of the bone.
These changes occurred about 3 months after the injury. This was the
largest group of patients in the series (47%). -
Group IV. Fragmentation and collapse of
the lunate, the result of one or more vertical fractures through the
area of rarefaction. This condition took at least 6 months to develop
and was seen in 32% of the patients. -
Group V. End-stage disease with secondary
arthritis. These patients were considerably older than those in the
other groups, and the duration of their disease was the longest. They
comprised 14% of the cases.
classification by correlating the radiographic appearance of the lunate
with the clinical picture. Lichtman classified Kienböck’s disease into
four stages (52):
-
Stage I. The earliest manifestation of
the disease characterized by a linear compression fracture on
conventional radiographs or CT. Since the introduction of MRI, it is
now possible to diagnose early vascular injury to the bone. Clinically,
patients complain of mild wrist pain. -
Stage II. Radiographically, the lunate
becomes more dense. Initially, the size and shape of the bone remain
the same, but later in this stage there is a decrease in the vertical
height of the bone on its radial aspect. This is an ominous
radiographic finding that usually indicates that further collapse can
be anticipated. Patients typically complain of local pain and
tenderness over the lunate. The wrist is sometimes swollen as a result
of joint synovitis. -
Stage III. The entire lunate bone is
collapsed in the frontal plane and elongated in the sagittal plane.
Bony deformation is commonly associated with alterations in the
architecture of other parts of the carpus; the capitate is shifted
proximally, there is scapholunate dissociation with rotatory
subluxation of the scaphoid, and the triquetrum is ulnarly deviated.
Lichtman later subdivided this stage into III-A and III-B. In stage
III-A, the rotatory subluxation of the scaphoid is not fixed, whereas
in III-B it is fixed. The extent of lunate collapse can be quantified
by the carpal height ratio. The ratio is the distance between the
distal articular surface of the radius and the base of the third
metacarpal, divided by the height of the third metacarpal. The normal
ratio is 0.54 ± 0.03 (61). Conventional radiographs often fail to show the extent of bone damage, and tomography is required (Fig. 42.15).
Patients’ symptoms in this stage are similar to those with stage II
disease, although they usually have greater wrist stiffness.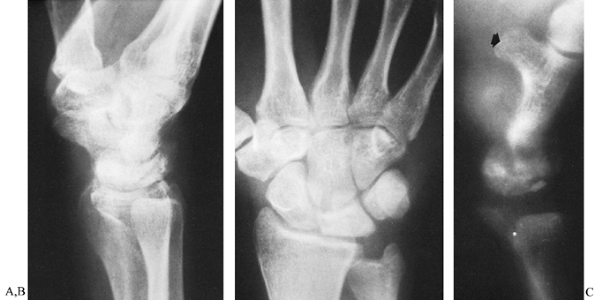 Figure 42.15. A,B:
Figure 42.15. A,B:
Conventional lateral and posteroanterior views of the wrist in a
patient with Kienböck’s disease and an ulna minus deformity. The
integrity of the lunate could not be determined by conventional
radiographs. C: Lateral tomography clearly
shows that the lunate had fragmented. This view also demonstrates the
usefulness of tomography for visualizing the hamate bone and its hook (arrow). -
Stage IV is end-stage Kienböck’s disease
with generalized arthritic changes in the wrist. Clinical findings can
range from mild discomfort to severe incapacitating pain, exacerbated
by physical activities. There is usually a significant loss of wrist
mobility.
on the stage of the disease. Although opinions differ regarding the
efficacy of a particular procedure, most agree that the objective of
treatment for the early stages of the disease is to prevent further
deterioration of the lunate and, if possible, reverse any changes that
have already occurred. Immobilizing the wrist is a treatment option,
but it is impractical because it must be prolonged, up to 1 year, and
there is no assurance that it will be successful. Generally, surgery is
the preferred treatment, and the procedures indicated for stages I and
II can be divided into two groups: decompression of the lunate, and
restoration of circulation to the bone.
-
Leveling the distal articular surfaces of the radius with the ulna when there is an ulna minus variance.
-
Shortening both radius and ulna when they are of equal length to decrease muscle forces across the wrist.
-
Changing the inclination of the articular surface of the radius by a lateral closing wedge osteotomy.
-
Intercarpal arthrodesis to transfer some
of the load to adjacent carpal bones (e.g.,
scaphoid–trapezial–trapezoid arthrodesis or scaphoid–capitate
arthrodesis) (63,100). -
Capitate shortening to decrease the load on the central column (3,4).
accomplished either by lengthening the ulna or by shortening the
radius. Each method has its advantages and disadvantages. Ulna
lengthening is less complicated but requires an iliac bone graft, which
adds donor site morbidity to the procedure. A more serious potential
problem is delayed or even nonunion at the interfaces of the ulna with
the intercalated graft. In addition, the plate necessary for fixation
of the graft is in a subcutaneous position and often must be removed
later. Another disadvantage of the procedure is that the lengthened
ulna may impinge against the sigmoid notch of the radius and cause pain
with forearm rotation. With radial shortening, the primary disadvantage
is that it requires a more extensive surgical exposure than ulna
lengthening. Bone healing is more assured with radial shortening,
however, and removal of the plate is usually unnecessary when it is
placed on the volar aspect. Another advantage of radial shortening is
that it produces relative lengthening of the extrinsic tendons, which
results
in additional reduction of force transmission across the wrist (103). Most surgeons recommend radial shortening using a volar operative approach.
Hori in 1979. He recommended curetting and bone grafting the lunate
followed by implantation of the second or third dorsal metacarpal
artery and vein. Inserting a pronator quadratus pedicle bone graft from
the volar surface of the radius has also been suggested (47) as well as simple curettage and cancellous bone grafting combined with external skeletal traction (106).
cancellous inlay bone graft inserted into a trough fashioned between
the lunate and triquetrum (Fig. 42.16). The
triquetrum with its intact blood supply nourishes the bone graft, which
serves as a scaffold for revascularization of the lunate. The technique
for preparing the trough across the lunotriquetral joint is similar to
preparing a trough across a scaphoid nonunion. The procedure is
applicable for early stage I and stage II disease. It can occasionally
be used for stage III-A disease, provided there is only a linear
fracture line in the lunate and the bone has not fragmented. This can
best be determined by CT imaging.
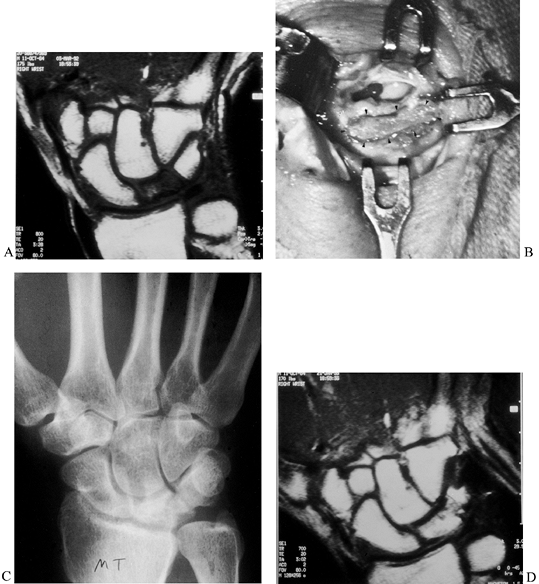 |
|
Figure 42.16. An MRI showing avascular necrosis of the lunate. B: A trough was made across the lunate–triquetral joint, and an autogenous iliac bone graft was inserted (arrows). C: Radiograph 4 months after surgery showing consolidation of the graft. D: MRI showing revascularization of the lunate
|
not seen until the later stages of the condition, when the bone has
fragmented and collapsed (stage III) and secondary arthritic changes
have developed (stage IV). Unlike treatment for stage I and stage II
disease, which is prophylactic and aims to prevent further
deterioration, treatment for stage III and stage IV disease is
palliative. The indication for surgery in patients with stage III and
stage IV disease is determined not by the severity of the radiographic
changes but rather by the magnitude of their symptoms. For stage III
disease, lunate excision and replacement with a silicone prosthesis was
a popular procedure before the mid-1980s. It is no longer recommended
because of the high incidence of particulate synovitis. A more
effective procedure with far fewer hazards is excision of the
fragmented lunate and intercarpal fusion. Scaphoid–trapezial–trapezoid
and scaphoid–capitate fusions used for reducing compressive forces on
the lunate in the early stages of Kienböck’s disease can also be used
as salvage procedures for stage III disease when combined with lunate
excision. With either type of intercarpal fusion, it is important to
insure that after the lunate is excised, the normal width of the space
previously occupied by the bone is maintained. An abnormally wide space
between scaphoid and triquetrum indicates that the scaphoid has
rotated, and if the scaphoid is arthrodesed in malposition it will
result in later arthritis at the radioscaphoid joint. Normal congruency
between scaphoid and capitate and between scaphoid and articular
surface of the radius must be preserved.
have developed, proximal row carpectomy and total wrist arthrodesis are
the most commonly performed salvage operations. Pain relief following
proximal row carpectomy is usually effective, probably on the basis of
denervation and decompression of the wrist joint (44).
Wrist motions are limited, but generally they are in a functional arc.
Grip strength is also reduced, but weakness can be minimized by
postoperative exercises. Total arthrodesis is the most predictable
operative procedure to provide a stable, pain-free wrist.
its hook (hamulus) with about equal frequency. Fractures through the
body are either in the sagittal plane or in the coronal plane (55,68,77).
Sagittal fractures can be divided according to which side of the hook
they lie on. Coronal fractures, when located near the dorsal surface of
the bone, are often associated with subluxations of the bases of the
fourth and fifth metacarpals (Fig. 42.17).
Regardless of direction, fractures through the body of the bone are
intraarticular, and it is important to determine if articular congruity
has been disrupted. Frequently, CT imaging is necessary. Displaced
fractures require open reduction and internal fixation.
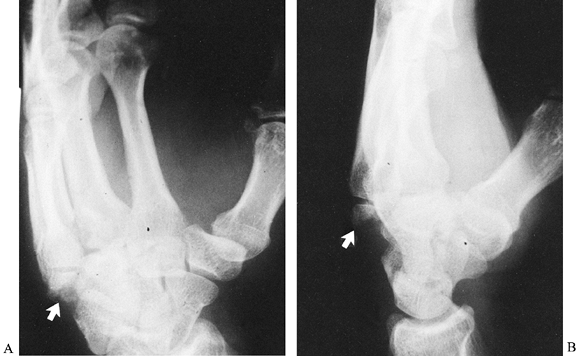 |
|
Figure 42.17. A,B: Oblique and lateral radiographs show a coronal fracture through the dorsal aspect of the hamate (arrows) associated with dorsal subluxations of the bases of the fourth and fifth metacarpals.
|
the hamate require a knowledge of the local anatomy. The hook serves as
attachment for the transverse carpal and pisohamate ligaments and is
the origin for two intrinsic muscles in the hypothenar eminence, the
opponens digiti quinti and the flexor digiti quinti. The motor branch
of the ulnar nerve passes close to the base of the hook on its ulnar
side. Fractures may therefore injure this nerve branch and cause
weakness of the ulnar innervated intrinsic muscles. Fractures may also
produce a sensory deficit on the palmar surfaces of the ring and little
fingers. Hook fractures are caused by direct or indirect trauma. Direct
trauma is probably the more common mechanism of injury and is often
associated with sports activities, such as baseball and tennis. When
athletes grip a baseball bat or tennis racquet with the butt end of the
handle resting on the hypothenar eminence, the force of the swing is
transmitted to the bone, which causes it to fracture. Frequently,
the athlete reports a painful “snap” or “crack” (8,88).
Indirect trauma to the hook occurs as a result of a fall on the palm
with the force transmitted to the bone through muscular and ligament
attachments.
wrist or in the hypothenar area of the palm. Tenderness directly over
the hook should be considered a fracture until proven otherwise.
Frequently, diagnosis is delayed because of inadequate radiographs. The
profile of the hook can not be visualized on routine views, although on
the PA view it can be seen as an oval density or “eye sign,” which
represents its junction with the body of the bone (Fig. 42.18) (67).
Absence of an “eye sign” should arouse suspicion of a fracture at the
base of the hook. If the sign is present, however, it does not exclude
a fracture through the middle of the hook or at its tip. Although
carpal tunnel
views
show the hook in profile, they often fail to show its base, which is
the usual location for most fractures. This is frequently the situation
following acute fractures because pain prevents patients from extending
their wrists sufficiently for the radiograph to profile the entire
hook. For a carpal tunnel view to be diagnostic, it must visualize the
flare at the base of the hook where it joins the body of the hamate.
Incomplete carpal tunnel views are sometimes interpreted as “negative,”
and only later is the correct diagnosis made. The radiographic
technique that is most effective for visualizing the hook is CT in the
lateral projection (Fig. 42.19).
 |
|
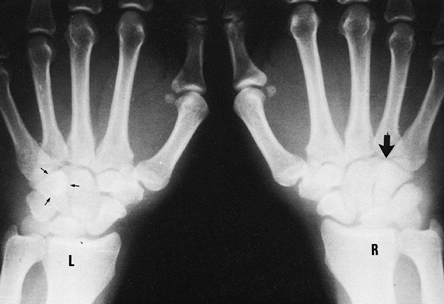
Figure 42.18. Posteroanterior radiograph of both hands. The oval density or “eye sign” representing the hook of the hamate (small arrows) is seen in the view of the left hand, but it is absent (large arrow) in the view of the right hand because it was fractured.
|
 |
|
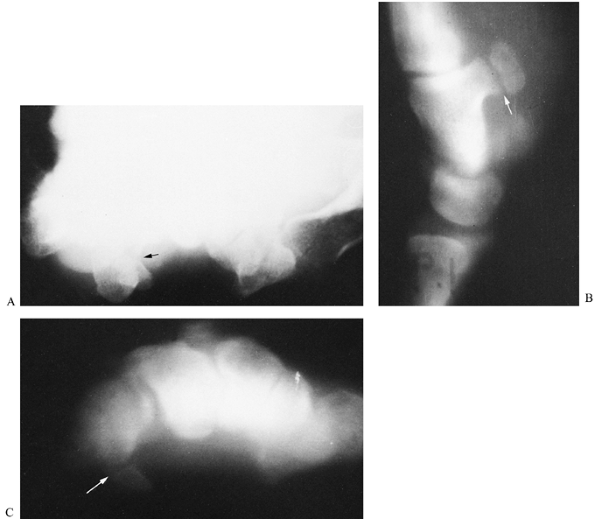
Figure 42.19. A:
Routine carpal tunnel view in a patient who complained of tenderness over the area of the hook of the hamate. Although there appears to be a disruption in the cortical outline of the hook of the hamate (arrow), the radiograph is not conclusive and was interpreted as negative. B: A lateral trispiral tomograph clearly demonstrates a fracture of the hook (arrow). C: The fracture was also evident on a carpal tunnel tomogram (arrow), but not with the same degree of clarity as was demonstrated on lateral tomography. Lateral tomography is the preferred imaging technique to visualize fractures of the hook of the hamate. |
is no displacement. When the parts are displaced, nonunion is likely
because the blood supply to the fragment has been disrupted (73). Although bone grafting has been suggested as a method of treatment for nonunion (99), excision of the fragment is the preferred treatment (16,84).
-
Make an incision along the radial border of the hypothenar eminence and curve ulnarly into the wrist flexion crease (Fig. 42.20). This incision avoids a tender scar over the eminence, which is an important contact area for grasp.
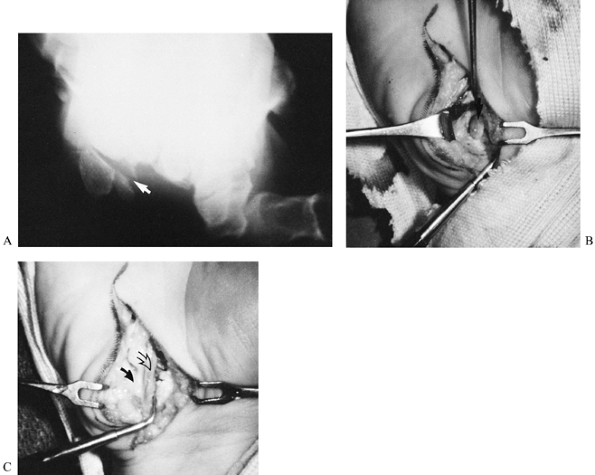 Figure 42.20. A: Radiograph of a fracture of the hook of the hamate in a 35-year-old golfer (arrow). B: At surgery, the hook (arrow) was stripped of its periosteum and of the ligaments and intrinsic muscles attached to it. C: After excision of the bone fragment, the soft tissues were closed (probe tip). Care must be taken during the procedure to identify and protect the ulnar neurovascular bundle (closed arrow, nerve; open arrow, vessels).
Figure 42.20. A: Radiograph of a fracture of the hook of the hamate in a 35-year-old golfer (arrow). B: At surgery, the hook (arrow) was stripped of its periosteum and of the ligaments and intrinsic muscles attached to it. C: After excision of the bone fragment, the soft tissues were closed (probe tip). Care must be taken during the procedure to identify and protect the ulnar neurovascular bundle (closed arrow, nerve; open arrow, vessels). -
Identify the ulnar neurovascular bundle and carefully retract it.
-
Excise the hook by sharply dividing its ligament and intrinsic muscle attachments.
-
Repair the fibrous origin of the intrinsic muscles to preserve their power.
-
Postoperatively, immobilize the wrist in
slight flexion for 2 weeks. Patients can usually resume sports
activities within 8 weeks, although there will usually be some
discomfort with firm grasp for several months. Residual weakness is
generally not a problem, even in professional athletes.
into which a tendon, the flexor carpi ulnaris (FCU), inserts. The
pisiform also serves as origin for the pisohamate and pisometacarpal
ligaments, which secure it to the hamate and bases of the fourth and
fifth metacarpals. The capsule between the pisiform and underlying
triquetrum is tough but lax (70).
following a fall on the palm. They also occur in individuals who use
the heel of their hand as a hammer for striking objects. These
fractures are best visualized by anterior-oblique and carpal tunnel
radiographs (Fig. 42.21).
 |
|
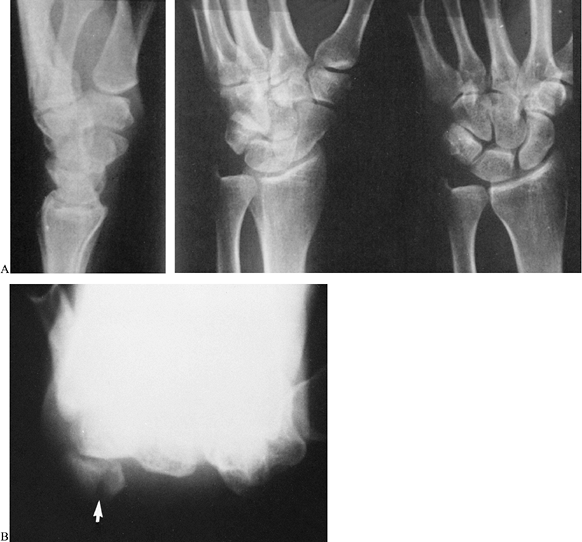
Figure 42.21. A:
A fracture of the pisiform was not evident on these three conventional radiographic views because of superimposition of other carpal bones. B: The fracture was obvious on a carpal tunnel view (arrow). |
conservative splinting. However, fragmentation can occur, which
requires excision of the bone. Chronic problems at the pisotriquetral
joint can also occur as a consequence of damage to the articular
cartilage resulting in chondromalacia. Occasionally, pain and local
tenderness
are so severe that excision of the pisiform is warranted (Fig. 42.22).
Excise it subperiosteally, preserving the fibrous attachments of the
ligaments and FCU, which are then resutured. Postoperatively, apply a
dorsal splint with the wrist in slight flexion for 2 to 3 weeks.
 |
|
Figure 42.22. A:
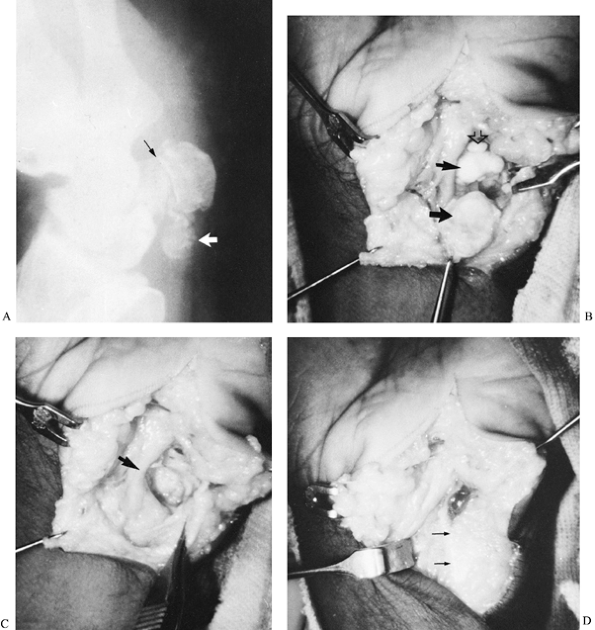
A 55-year-old woman complained of chronic pain over the pisiform area of her right palm. She reported falling on the heel of her hand approximately 1 year earlier. The oblique radiograph showed narrowing of the pisotriquetral joint (black arrow) with calcification in the area of the flexor carpi ulnaris (white arrow). B: At surgery, there was complete erosion of the cartilage over the contiguous articular surfaces of both bones (small arrow, triquetrum; large arrow, pisiform). An osteochondral body (open arrow) is seen at the distal end of the triquetrum. C: View after excision of the pisiform. The forceps is grasping the flexor carpi ulnaris tendon, and the ulnar neurovascular vesicle (arrow) is to the radial side of the triquetrum. D: The capsule of the pisotriquetral joint was sutured to the edge of the flexor carpi ulnaris (arrows) to preserve its function. |
The low incidence is related to the anatomic position of the bone,
which is surrounded and securely fixed to the trapezium, base of the
second metacarpal, capitate, and scaphoid. Dislocations are more likely
to occur than fractures, but they are also very rare.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
BD, Frykman GK, Taleisnik J. Treatment of Scaphoid Nonunion with Cast
and Pulsed Electromagnetic Fields. A Study Continuation. J Hand Surg 1982;17A:910.
TH. Certain Points in the Anatomy and Mechanism of the Wrist Joint
Reviewed in the Light of a Series of Roentgen Ray Photographs of the
Living Hand. J Anat Physiol 1895;31:59.
RD, Shives TC, Dobyns JH, Linscheid RL. Kienböck’s Disease: The Natural
History of Kienböck’s Diseases and the Consideration of Lunate
Fractures. Clin Orthop 1980;149:98.
R. Concerning Traumatic Malacia of the Lunate and the Consequences:
Degeneration and Compressive Fractures. Peltier LF (trans). Clin Orthop 1980;149:4.
RL, Dobyns JH, Beabout JW, Bryan RS. Traumatic Instability of the
Wrist. Diagnosis, Classification and Pathomechanics. J Bone Joint Surg 1972;54A:1612.
RL, Youm Y, Flatt AE. Kinematics of the Wrist. I. An Experimental Study
of Radial-Ulnar Deviation and Flexion-Extension. J Bone Joint Surg 1978;60A:423.
JS, Gelberman RH, Taleisnik J, Baumgaertner M. The Arterial Anatomy of
the Human Carpus. Part II: The Intraosseous Vascularity. J Hand Surg 1983;8A:375.
P, Wright TW, Wallace PF, Dell PC. Excision of the Hook of the Hamate:
A Retrosprective Survey and Review of the Literature. J Hand Surg 1988;13A:612.
F. On Lunatomalacia (Kienböck’s Disease): A Clinical and
Roentgenological Study, Especially on Its Pathogenesis and the Late
Results of Immobilization Treatment. Acta Chir Scand [Suppl] 1947;126:1.
RM, Gelberman RH, Evans EF. Scaphocapitate Fractures. Patterns of
Dislocation, Mechanisms of Injury, and Primary Results of Treatment. J Bone Joint Surg 1980;62A:271.