Lumbar Spondylo-Listhesis
meaning “movement or slipping.” Spondylolisthesis describes the
pathologic state of one vertebra slipping on another; this can be
forward (anterolisthesis) or backward (retrolisthesis). In the lumbar
spine, the etiology of spondylolisthesis is varied. Spondylolysis, a term that comes from the Greek spondylo plus lysis,
or “break,” indicates a defect in the pars interarticularis region.
Spondylolisthesis can occur after the development of a lytic defect,
after degeneration of the intervertebral disc and facet joints, or from
a variety of other causes.
the 18th century. A large body of literature exists that attempts to
describe the pathogenesis, natural history, and treatment of this
extremely common disorder. This chapter describes the classification of
spondylolisthesis and summarizes the important aspects of the clinical
presentation and treatment options of the various types of lumbar
spondylolisthesis. The focus is on the two most common types of
spondylolisthesis—isthmic and degenerative. The most well-known system
of classifying lumbar spondylolisthesis evolved from the cooperative
endeavors of three prominent surgeons, Wiltse, Newman, and Macnab. The Wiltse classification, as it is commonly known, divides the disorder into five types (Fig. 18-1):
-
Type I—dysplastic or congenital. This type is due to the presence of congenital abnormalities of the upper sacrum or the posterior arch of L5.
-
Type II—isthmic.
The lesion is in the pars interarticularis. Three types can be
recognized: IIA, lytic-fatigue fracture of the pars; IIB, elongated but
intact pars; and IIC, acute fracture of the pars. -
Type III—degenerative. This type is due to long-standing instability, arthritic changes, and degeneration of the disc and facet joints.
-
Type IV—traumatic.
Acute fractures in areas of the bone hook other than the pars (often
the pedicles) allow translational instability of the motion segment. -
Type V—pathologic. There is generalized or localized bone disease (metabolic or neoplastic).
-
Type VI (this type has since been added by convention)—iatrogenic.
This is due to aggressive resection of the facet joints or
intervertebral disc, or both, leading to the creation of instability in
the lumbar spine.
determining the etiology of spondylolisthesis. In the normal lordotic
posture of the lumbar spine, axial load is shared between the
intervertebral discs (approximately two thirds) and the facet joints
(one third). This distribution can vary depending on posture and
loading conditions. The oblique orientation of the facet joints results
in limitation of axial and sagittal rotations within the lumbar spine.
This facet angle changes from a relative sagittal orientation in the
upper lumbar spine to a more coronal orientation in the lower lumbar
vertebrae. Most importantly, in the lower lumbar spine, the facet
joints provide significant resistance to anterior shear in flexion,
especially with increased lumbar lordosis. At the level of L5-S1, the
strong iliolumbar ligaments provide additional restraint to forward
flexion and lateral motion. This restraint may cause stress
concentration at the superjacent (L4-5) motion segment.
performing a comprehensive analysis of the etiologic factors involved
in spondylolisthesis. His theories formulated many of the concepts that
since have withstood critical analysis. The normal lordosis of lumbar
spine in animals that walk erect leads to a constant downward and
forward thrust to the lower lumbar vertebrae. This tendency to forward
slipping is counteracted by the facets, pedicles, neural arches, and
normal bone structure. These anatomic observations form the basis for
the Wiltse classification system: the slip may occur secondary to a
defect in the facets, a defect in the neural arch or pedicle, or from a
structural inadequacy of bone.
the pars, the morphologic changes in the facets that accompany
arthritis, and the predisposing factors in each case. These studies are
reviewed in subsequent sections.
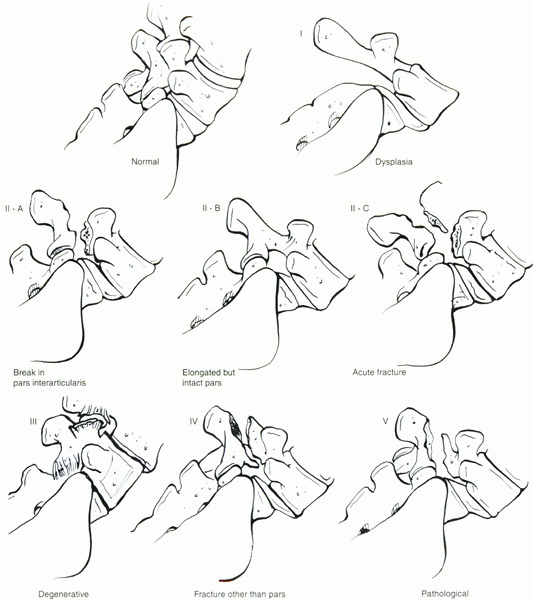 |
|
Figure 18-1 Schematic drawing of the modified Wiltse classification of lumbar spondylolisthesis.
|
congenital form of spondylolisthesis. There is a 2:1 female-to-male
ratio. These slips account for approximately 14% to 21% of all
spondylolisthesis cases. These congenital abnormalities of the
lumbosacral junction, including dysplasia of the fifth lumbar and
sacral neural arches and facets, compromise the normal buttress
function of the posterior elements. Gradual forward displacement of the
fifth lumbar vertebra on the sacrum occurs. Displacement is early but
usually does not progress beyond 50%.
may elongate or separate. An elongated pars is difficult to distinguish
from isthmic subtype B on plain radiographs (see later); a broken pars
may be difficult to distinguish from subtype A. These may be discerned,
however, during surgery.
in most of these patients, two phenomena are observed. The slip is
limited in its extent because of impingement of the neural arch on the
anterior structures. At the same time, significant neurologic findings
are evident with relatively low-grade slips (25% to 35%), resulting
from the compression of the cauda equina by the posterior arch of L5 as
it is carried forward with the body of L5.
-
In subtype A,
the dysplastic facets have a transverse, or horizontal, orientation.
Spina bifida of L5 is common, and early symptoms of the slip, such as
hamstring tightness, are relatively common. Fusion for this subtype
often is required because of the unstable nature of the motion segment. -
In subtype B,
the facets have an asymmetric sagittal orientation, and the neural arch
is likely to be intact. Associated symptoms of hamstring tightness, leg
pain, and even cauda equina syndrome are common. Management requires
decompression and fusion for the best clinical outcome. -
Subtype C
groups other causes of congenital malformations of the lumbosacral
junction. Treatment is based on the nature of the abnormality and the
severity of clinical symptoms.
defect in the pars interarticularis (with normal facets) that permits
the forward slippage. It is the most common type of spondylolisthesis.
Isthmic spondylolisthesis is divided into three subtypes (Fig. 18-2):
-
Spondylolytic fatigue fracture of pars
-
Elongated but intact pars
-
Traumatic (acute pars fracture)
males predominate in a 2:1 ratio. Much attention has been devoted to
the incidence and etiology of this type of spondylolisthesis. It is
most common at the L5 level, leading to L5-S1 slip.
isthmic spondylolisthesis. Relatives of patients have a reported
incidence of 28% to 69%, which is much higher than in the general
population. It is rare in individuals of African descent (2.8%), higher
in individuals of northern European descent (6.4%), and highest in
Alaskan Inuits (26% to 50%). This high incidence is thought to be due
to a combination of genetic and environmental causes; Alaskan natives
have been observed to stoop over while harvesting seal blubber,
possibly changing the stresses on the lumbar spine. The incidence
continues to increase in this population until age 34, in contrast to
other populations, lending evidence for an environmental contribution.
studies, it is reported only after walking begins. It has not been
reported in quadrupeds or chronically bedridden adults. The incidence
increases dramatically between age 5 years and adolescence. Wiltse
found a 5% incidence of spondylolysis in children 5 to 7 years old,
increasing to 6% to 7% by age 18. Patients with Scheuermann’s kyphosis
and certain athletes seem to have a higher incidence. Football linemen,
gymnasts, butterfly swimmers, weight lifters, rowers, divers,
wrestlers, and tennis players all have been noted to use excessive
lumbar extension, contributing to the theory of a fatigue fracture.
Wiltse defined the basic lesion in isthmic spondylolysis as a fatigue
fracture of the pars, and most authors are in accord.
-
There seems to be a genetic predisposition or diathesis.
-
Mechanical stresses from environmental or
occupational factors influence the development of a type of fatigue
fracture in susceptible or high-risk individuals. -
These fatigue fractures develop at an
earlier age, after relatively minor trauma, and persist relative to
other known fatigue fractures. -
The presence of a lytic defect adds to
instability and contributes to the development of a spondylolisthesis
to a varying degree. -
Females are more prone to severe
displacement and more frequently need stabilization to treat their
instability and lessen their symptoms. -
Less than 20% of patients with a spondylolysis develop symptoms of any kind related to the spondylolysis.
treatment, without undergoing olisthesis. The percentage of patients
with spondylolysis who develop spondylolisthesis was evaluated in a
longitudinal study by Fredrickson et al.
They found that in patients with lytic defects of the pars, most slips
occurred before adulthood: 68% of 5-year-old children had an associated
slip, increasing only slightly to 74% in adulthood. Saraste followed
255 patients with isthmic spondylolisthesis for 20 years and found that
40% of adults did not have progression, 40% progressed less than 5 mm,
and only 15% progressed more than 1 cm. Slip progression has been found
to correlate with a poor outcome in terms of pain and deformity. The
literature supports the conclusion that low percentages of slips
progress, but there is little agreement as to which factors predict
progression. In general, isthmic slips that progress do so farther than
degenerative slips, which are limited by the bone anatomy.
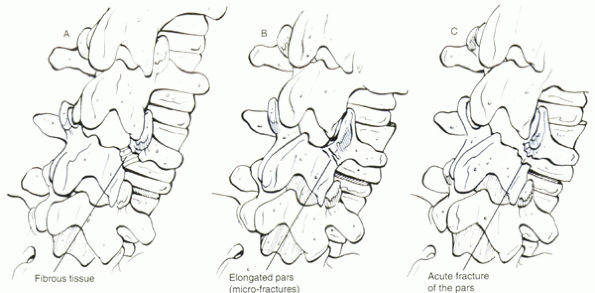 |
|
Figure 18-2 Isthmic spondylolisthesis subtypes: (A) Spondylolytic fatigue fracture of pars. (B) elongated but intact pars. (C) traumatic (acute pars fracture).
|
later), have been reported to predict progression by some authors but
not by others. Floman, echoing Taillard, postulated that slip
progression after skeletal maturity almost always is related to disc
degeneration at the slip level. Slip progression in his study always
was accompanied by disc degeneration at the level below the pars
defect. The presence of this pathology in the disc may explain the late
onset of symptoms associated with the slip. Wiltse pointed out,
however, that when the disc collapses, there is little further
progression.
childhood or early adolescence, symptoms are relatively uncommon in
children. Teenagers and young adults may experience a dull aching back
pain, which is aggravated by athletic activity, particularly repetitive
flexion/extension motion. This pain may radiate down into the buttocks
or posterior thighs. Radicular pain is uncommon in adolescents and more
common in adults, and this is usually in the L5 distribution owing to
nerve compression by the hypertrophic callus at the pars defect or
owing to the foraminal stenosis accompanying the spondylolisthesis,
commonly at the L5-S1 level. Objective signs on examination include
postural changes, such as a flattening of the buttocks and increased
lumbar lordosis. A waddling gait may reflect vertical rotation of the
pelvis and short steps due to an inability to extend the hips. The
characteristic crouched gait is seen more commonly in high-grade slip
with associated hamstring tightness. A step-off at the lumbosacral area
may be evident. Uncommonly, there is weakness of the extensor hallucis
longus muscle secondary to compression of the fifth nerve root.
Compression of this root is an important concept; the root is
compressed by the fibrocartilaginous material at the site of the pars
defect, made worse by the stretching from the slip (Fig. 18-3).
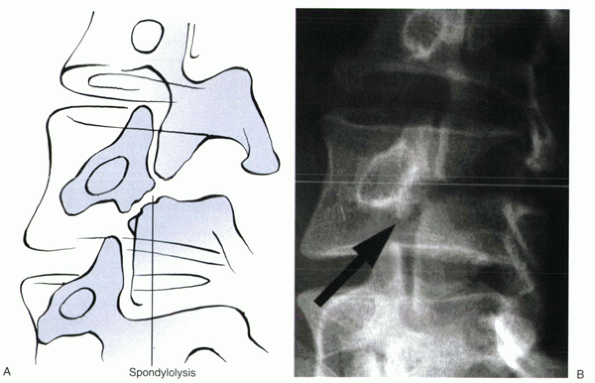
anteroposterior, lateral, and oblique projections. The defect is seen
as the “collar” on the “Scotty dog” (Fig. 18-4).
In patients with a defect, collimated lateral views show it 84% of the
time. Oblique views are controversial; one study reported that they
show a lytic defect in less than 4% of cases not seen on the lateral
view, with more than twice the gonadal radiation. Further imaging to
identify a lytic defect should be with a bone scan using single-photon
emission computed tomography (SPECT) (Fig. 18-5).
SPECT also can help determine whether a defect is acute or chronic. To
maximize the chances of observing an olisthesis, standing lateral
radiographs should be obtained.
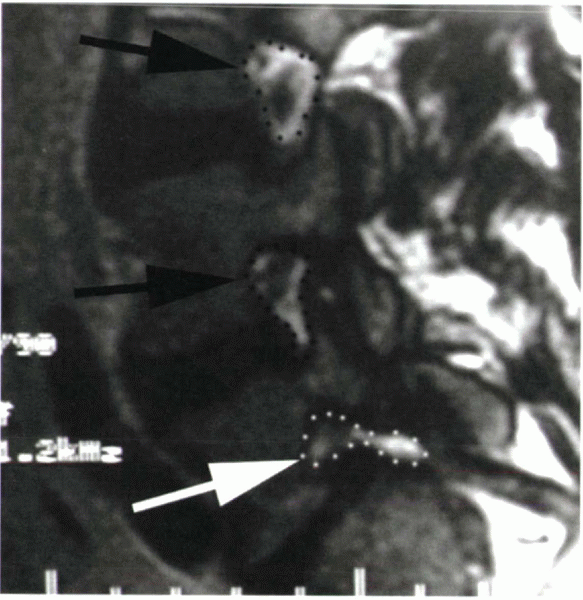 |
|
Figure 18-3 T1-weighted sagittal MRI of a patient with isthmic spondylolisthesis at L5-S1. Note the open intervertebral foramen (black arrows and outlines), in contrast to the narrowed foramen at L5-S1 (white arrow and outline). Compression of the exiting L5 nerve root is shown.
|
 |
|
Figure 18-4 Illustration (A) and oblique radiograph (B) of a lytic pars defect classically described as a collar on the “Scotty dog.”
|
 |
|
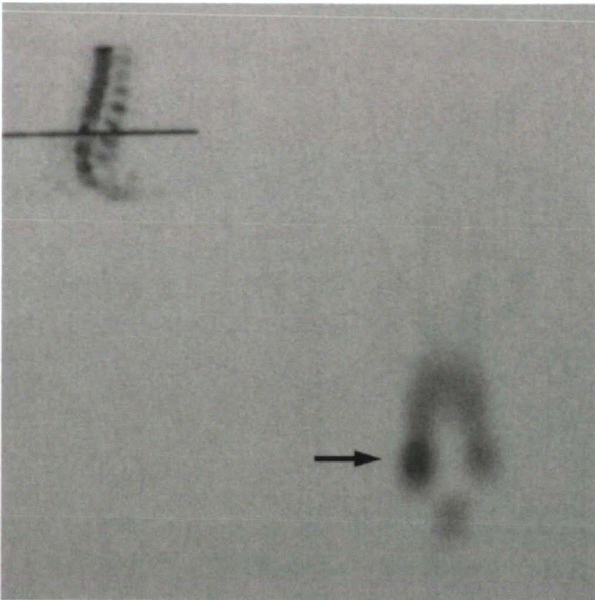
Figure 18-5 SPECT with uptake at the site of a lytic pars defect.
|
 |
|
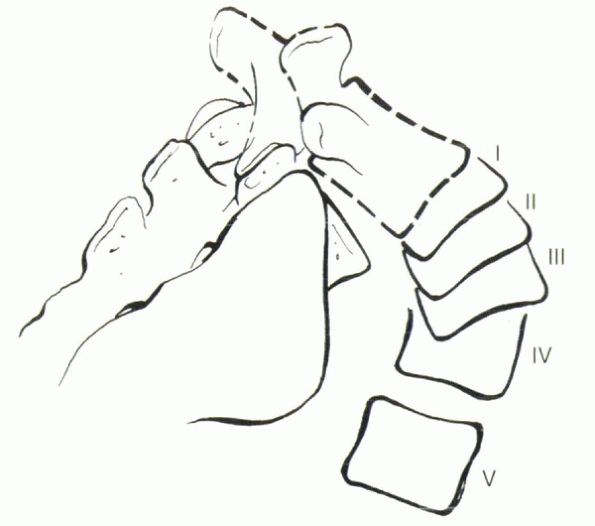
Figure 18-6
Schematic drawing of the Meyerding classification of spondylolisthesis, based on the percentage of slippage of the vertebral bodies (I = 0% to 24%, II = 25% to 49%, III = 50% to 74%, IV = 75% to 99%). Stage V is 100% olisthesis, or spondyloptosis. |
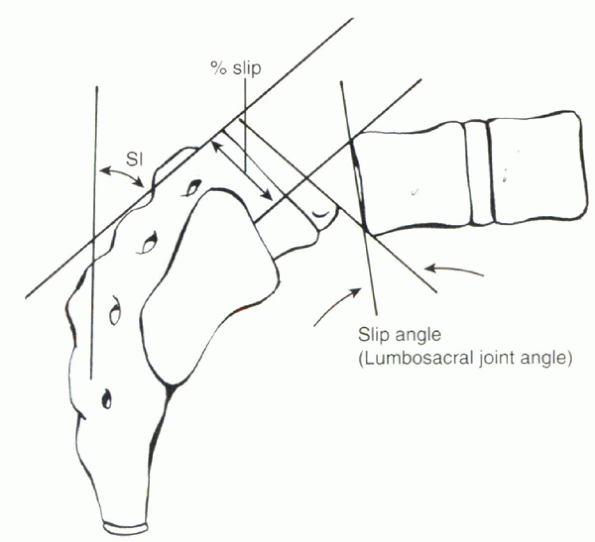
Wiltse and other authors, using a standard lateral radiograph in the
standing position. The two most common measurements are the grade of
the slip and the slip angle. The Meyerding grading classification is
used most commonly to express the slip as a percentage of the
anteroposterior measurement of the body of S1 (Figs. 18-6 and 18-7).
This classification divides the sacrum into quadrants I through IV and
expresses the grade of the slip according to how far the body of L5 has
slipped forward on the sacrum. Grade V is used for spondyloptosis, or
100% slip. The slip angle, the angle formed by lines drawn along the
posterior border of the sacrum and along the anterior body of L5,
measures the kyphosis associated with the deformity (see Fig. 18-7).
The slip angle has been found by some to predict progression of the
slip. In particular, Saraste found that risk factors for slip
progression included dysplastic etiology, slip angles greater than 40
degrees, Meyerding grades III or higher, female patients, and the
presence of a rounded-off or domed S1.
 |
|
Figure 18-7 Calculation of the slip angle as a measure of kyphosis and of the percent slip of one vertebral body on the other.
|
or spondylolisthesis range from observation, restriction of athletic
activities, bracing or casting, and open repair of a pars defect to
decompression, fusion, and possible reduction of the slip. The natural
history of these disorders is paramount in selecting the best treatment
option; many of these patients, particularly adults with the disorder,
do not require active treatment, much less operative intervention, for
a successful outcome. Adults with continued back pain and L5 radicular
symptoms can be treated effectively with a decompression and fusion
procedure.
-
Incidental pars defect—observe with radiographs annually in a growing child; no restrictions
-
Up to 25% slip—no limitation in activity; observe with radiographs semiannually
-
Up to 50% slip in an asymptomatic patient—observe
with radiographs semiannually; consider restriction of high-risk
athletic activity and avoiding an occupation with heavy labor -
Up to 50% slip in a symptomatic patient—conservative
treatment with activity modification, physical therapy, or
bracing/casting (particularly if SPECT documents an acute pars
fracture); observe with radiographs semiannually until maturity, then
annually -
Greater than 50% slip—consider surgical intervention
intervention include persistence of major symptoms for an extended
period despite conservative care, postural symptoms/deformities not
relieved by physical therapy, progressive neurologic deficit,
progressive slipping beyond 25% to 50%, and a high slip angle in a
young patient (because this indicates a high likelihood of slip
progression). Repair of the pars defect in cases of spondylolysis
without slip has been advocated. Bradford and Iza reported good results
with bone grafting and fixation using wiring between the transverse and
spinous processes. Other techniques involve direct screw fixation and
bone grafting of the defect. The advantage of these techniques lies in
preserving the affected and adjacent motion segment as long as no
olisthesis is present. In an older patient with a spondylolisthesis,
the competency of the intervertebral disc must be questioned, however,
and a fusion
is a better operation. Pars repair is contraindicated when a slippage has occurred.
patients with low-grade slips unresponsive to conservative care. As
described by Wiltse, this fusion can be performed through a midline
incision, bilateral lateral fascial incisions, and a paramidline
muscle-splitting approach to the spine. Abnormal motion is eliminated,
but decompression is not performed with this technique.
Postoperatively, patients should be braced with a lumbosacral orthosis
or body cast with a leg extension to improve fusion rates. The role of
more aggressive surgical techniques also has been explored. If more
than 50% slippage is present, many authors advocate extension of the
fusion to L4 owing to the technical difficulty of accessing the L5
transverse process without exposure of the L4 process. Extension to L4
also improves the success of fusion by increasing the surface area for
the fusion mass.
controversial. If significant radicular symptoms exist, most authors
advocate resection of the hypertrophic fibrocartilaginous mass at the
pars defect, decompressing the L5 root. The basis of the decompression
is the Gill procedure. Gill described removal of the loose lamina, with
further resection of the pars area as above. Wiltse, using a midline
exposure, believed initially that this did not cause further
instability. After observing slip progression in this patient
population postoperatively, Wiltse later changed to his paramidline
approach and included a fusion in the operation. Rates of slip
progression after decompression without fusion have been reported to be
27%, particularly in patients younger than 30 years old. Wiltse went on
to report that most radicular symptoms from compression of the L5 root
and the associated back pain and hamstring tightness completely resolve
with a successful fusion.
of symptomatic adult isthmic spondylolisthesis have been shown to be
equivalent to posterolateral fusion with instrumentation. These and
other observations indicate that the motion segment instability causes
irritation of the nerve root, rather than compression. We routinely add
a transforaminal lumbar interbody fusion to the normal posterolateral
fusion in these patients to maximize success of a solid arthrodesis. In
the absence of severe neurologic injury (cauda equina syndrome,
significant motor weakness), decompression is not necessary and may
worsen results. In a prospective, randomized trial comparing
single-level fusion with or without decompression for isthmic
spondylolisthesis in adults, Caragee found that the addition of
decompression to arthrodesis significantly increased the pseudarthrosis
rate and led to more unsatisfactory results. Although it is tempting to
compensate for the instability caused by decompression using
instrumentation as an adjunct to fusion, Caragee did not find any
decrease in the pseudarthrosis rate with the addition of pedicle screw
fixation.
controversial, and to some extent this is tied up in the question of
whether a reduction should be performed. The use of pedicle screw
instrumentation is extremely common in these patients. It may be used
to stabilize the fusion, obviating postoperative bracing, or to
maintain or achieve a reduction on the operating table. Moller and
Hedlund conducted a prospective randomized trial of instrumentation in
the treatment of adult isthmic spondylolisthesis and found that
supplemental pedicle screw fixation prolonged the operative time and
increased total blood loss but did not affect the clinical outcome or
fusion rate.
reduced pseudarthrosis rate (owing to increased surface area for
fusion), a decrease in the rate of slip progression postoperatively,
preserved motion segments, and reduced clinical deformity. Most modern
systems require extension to L4 to pull the L5 vertebral body (via its
pedicle screw) backward in the sling created by the screws in S1 and
L4. The clinical outcome has not been shown to improve after these
reductions, however, and the complication rates are higher than
reported for in situ fusions, albeit with the use of older reduction
techniques. In a retrospective review of patients who underwent
reduction of high-grade slips, Hu et al found
overall good clinical results but a 25% significant complication rate.
Another retrospective study of patients treated with posterior
reduction, interbody fusion, and segmental fixation reported a high
fusion rate, no loss of reduction, and minimal complications. It
remains to be shown whether the newer techniques of reduction decrease
the complication rate for this part of the procedure.
formal reduction rarely is indicated. Bradford listed the following
criteria for patients who may be candidates for an attempted reduction:
-
Vertebral slippage greater than 60%
-
Slip angle greater than 50 degrees
-
Symptoms uncontrollable by nonoperative means
-
Age between 12 and 30 years
There has been no study in adults, however, documenting an improved
clinical outcome with reduction of the slip. In our practice, we
routinely combine a transforaminal lumbar interbody fusion with
decompression and posterolateral fusion. A partial reduction often is
obtained during preparation of the interbody space and fusion
procedure; further reduction is not attempted routinely, unless
significant external deformity exists or there is greater than a grade
II olisthesis.
to 25%, with most studies reporting fewer than 15% but a higher rate
with more severe slips. The rates of slip progression seem to be higher
after a Gill procedure and disruption of the posterior ligamentous
complex. Neurologic complications have been chiefly in the form of
radicular symptoms after reduction of high-grade slips, most commonly
from the root of L5. Complete reduction is not necessary, and because
most strain in L5 occurs in the last stages of reduction, this should
be avoided. Attention instead should focus on correcting the
lumbosacral kyphosis, which may decompress the nerve root. Cauda equina
syndrome also has been reported, with or without reduction of the slip.
This condition affects predominantly older individuals, after age 40,
and is thought to derive from a combination of facet arthritis and
degenerative disc disease. It occurs most often at the L4-5 level. In a
landmark study, Rosenberg reported on the incidence and predisposing
factors in 20 cadavers and 200 patients with degenerative
spondylolisthesis. This condition occurs five to six times more
commonly in women and three times more often in individuals of African
descent. The predominance in women is thought to be due to the
influence of a generalized ligamentous laxity. The increased incidence
in blacks is thought to be related to anatomic factors, which include
less lumbosacral lordosis than in other populations and a high
incidence of sacralization of L5.
involves an increase in stress borne by the posterior elements as a
result of degeneration of the intervertebral disc combined with
abnormal stress concentration on the L4-5 motion segment. Facet
morphology may influence development of slips. In contrast to the
coronal orientation at L5-S1, the facets at L4-5 have a tendency toward
a more sagittal alignment. This alignment decreases the facets’ ability
to resist forward flexion forces, increasing the tendency to slip and
accelerating facet arthritis. When disc degeneration occurs, the motion
segment settles into an anterolisthesis; this rarely progresses past
grade II and in general is less than that seen in isthmic slips.
spondylolisthesis is intertwined closely with that of spinal stenosis,
which commonly accompanies a degenerative spondylolisthesis. Patients
typically complain of lumbago-type low back pain that commonly radiates
into the buttocks or lateral thighs, proximal weakness or “drop spells”
because they are prone to collapse while walking, and intermittent
claudication symptoms. These claudication symptoms notably are not
relieved simply by standing still; patients often describe the need to
sit down or lean on something (classically the shopping cart) to
relieve the pain. This lumbar flexion effectively increases the
diameter of the spinal canal and intervertebral foramen, which relieves
the pressure on the nerves or cauda equina. This is a useful
distinction in assessing any contribution by vascular causes; vascular
claudication usually occurs after a repeatable distance and is relieved
by stopping and standing still.
usually corresponding to L5. Compression of the L5 nerve root is common
with degenerative spondylolisthesis. This compression results from
overgrowth of the facet at the L4-5 level, compressing the traversing
nerve root posterolaterally between the superior facet and the
posterosuperior border of the L5 vertebral body (the lateral recess).
the clinical examination in patients with degenerative slips often
reveals normal or even hypermobility of the lumbar spine in flexion,
with a notable lack of stiffness. This phenomenon is thought to be
related to a generalized ligamentous laxity in these patients, which
predisposes them to a slip. Extension maneuvers may exacerbate the pain
owing to narrowing of the canal and foramina. Cauda equina syndrome is
rare with this disorder but a history of such should be solicited
aggressively because the onset can be insidious, and urinary continence
issues, such as hesitancy, dribbling, and poor control, can overlap
with common genitourinary conditions in this age group.
which can show a spondylolisthesis not detected on supine films in 15%
of patients. Flexion/extension lateral radiographs may show a dynamic
slip, but this is relatively rare and may not influence treatment.
Axial imaging by computed tomography (CT), with or without myelography,
traditionally has been the imaging modality of choice for degenerative
slips associated with spinal stenosis. CT gives excellent detail of the
source of compression, facet joint morphology, and pedicle orientation
and an idea of bone stock present. Magnetic resonance imaging (MRI) is
used increasingly for this disorder, often in combination with
CT-myelography, to evaluate the soft tissue structures further. MRI
reveals nerve root compression, disc pathology, facet joint synovial
cysts, yellow ligament hypertrophy,
and
other soft tissue sources of compression that may not be apparent on CT
or CT-myelography. MRI is a noninvasive imaging modality, avoiding
painful lumbar or cervical injections and the side effects of
myelography (e.g., headache and nausea), which can occur in 20% of
patients.
 |
|
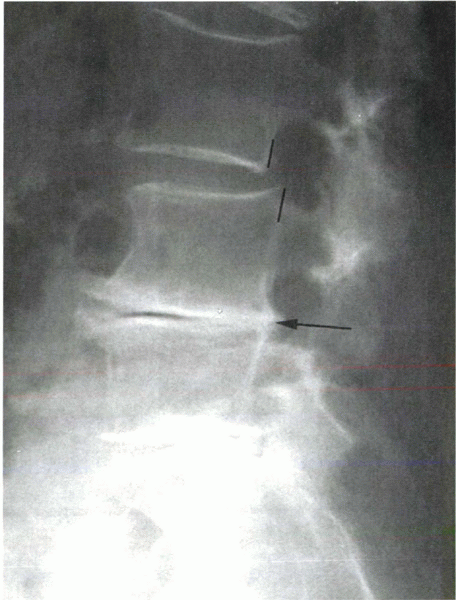
Figure 18-8
Standing lateral x-ray showing a degenerative spondylolisthesis at L3-4, above a relatively stiff degenerative segment at L4-5 (arrow). |
degenerative spondylolisthesis call attention to the fact that this
disorder may not be as aggressive as previously thought. About 25% to
30% of patients may experience progression of the slip, but rarely to
more than 30% of the subjacent vertebra. Progression of the slip does
not correlate with clinical symptoms.
radicular symptoms associated with degenerative spondylolisthesis is
rest not to exceed 1 to 2 days, nonsteroidal antiinflammatory drugs,
and activity modification. This supportive therapy is supplemented by
physiotherapy emphasizing flexion exercises and back strengthening,
progressing to aerobic conditioning. Use of a stationary bicycle is
encouraged; the seat and handlebars can be set up to allow lumbar
flexion during aerobic conditioning, expanding the canal and neural
foramen and allowing greater exercise tolerance with less irritation of
nerve roots. We also routinely use epidural steroid injections for this
disorder, similar to the treatment of spinal stenosis without a slip.
These injections are more effective for relief of radicular-type pain
and less effective for back pain.
-
Persistent or recurrent leg pain despite a minimum of 3 months of conservative treatment
-
Progressive neurologic deficit
-
Significant reduction in the quality of life
-
Confirmatory imaging studies consistent with the clinical findings
decompression, decompression and posterolateral fusion with or without
instrumentation, or anterior or posterior interbody fusion.
reported results of 47 patients with degenerative spondylolisthesis who
underwent decompression with or without fusion. They found that
patients who had a radical decompression fared poorly; patients who
underwent spinal fusion fared the best. Poor results from these and
other authors after decompression alone are related to the progression
of slip in these patients postoperatively and to the persistence of
instability at the level. Approximately 25% to 50% of patients who
undergo decompression without fusion experience a progression of their
slip; this postoperative progression seems to correlate variably with
outcome. Bone regrowth after decompressive laminectomy in patients with
degenerative slips also is higher in patients who are not fused,
contributing to recurrence of symptoms.
fusion, with or without instrumentation, persists despite several
prospective, randomized trials examining these variables in the
treatment of degenerative spondylolisthesis. In 1991, Herkowitz and Kurz
published their results in patients with associated spinal stenosis.
They showed statistically better clinical outcomes in patients who
underwent fusion. Zdeblick compared noninstrumented fusions with
semirigid and rigid instrumented fusions in 124 patients, 56 of whom
had spondylolisthesis, and found better fusion rates in the rigidly
instrumented group. Better clinical results also were found in the
instrumented groups. Bridwell reported on 44 patients divided into
three groups: (1) no fusion, (2) noninstrumented posterolateral fusion,
and (3) instrumented posterolateral fusion using pedicle screws.
Instrumentation significantly improved fusion rates, functional
outcome, and sagittal alignment.
spondylolisthesis was performed in 1994 (Mardjetko et al). This study
examined the issues of fusion and the use of instrumentation as an
adjunct. In patients undergoing decompression without arthrodesis, 69%
had a satisfactory outcome. Progressive slipping after decompression
was noted in most reports. The addition of arthrodesis increased the
satisfactory outcome to 90%, with 86% achieving solid fusion. In this
meta-analysis, five studies were identified as suitable for examining
the value of instrumentation; the authors reported a strong trend (p = 0.08) toward increased fusion with pedicle instrumentation, but minimal difference in clinical outcome.
randomized trials examining these issues have been published. The Volvo
award winner for 1997 (Fishgrund et al.) examined instrumentation in
this population; the authors found a significantly higher fusion rate
in the instrumented group (82% versus 45%), but no significant
difference in clinical outcomes with the use of instrumentation. They
concluded that at least in the short-term, successful arthrodesis does
not influence patient outcome. Later, with longer follow-up, the
patients with pseudarthrosis did not do as well, however, as the
patients with a solid fusion. This finding prompted the authors to
advocate the routine use of instrumentation to increase the fusion
rates. Another Volvo award winner (Thomsen et al., 1997) studied
posterolateral spinal fusions for spondylolisthesis or “segmental
instability.” The authors found that if neural decompression was
performed, instrumentation significantly improved functional outcomes.
Other comparisons between the instrumented and noninstrumented groups,
including fusion rates, did not show significant differences. They
observed “significant symptoms related to misplacement of a screw” in
4.8% of patients and concluded that routine use of pedicle screw
fixation alone is not justified as an adjunct to posterolateral lumbar
fusion.
spinal stenosis. A long trial of conservative therapy and injections
should be granted the patient before operative intervention is
undertaken. Surgery should consist of decompression and fusion. The use
of pedicle instrumentation should be tailored to the instability and
the extent of decompression. We believe that the use of instrumentation
in patients at risk of instability or pseudarthrosis is warranted to
increase fusion rates and improve stability, in turn improving outcome.
are rare. Wiltse noted that forced marches in recruits laden with gear
have been known to produce pars fractures and to generate a typical
“fluffy callus” at the site that almost always heals. He also noted
several cases of traumatic spondylolisthesis, all with an easily
recognizable history—of falling backward on a curb, with a blow to the
lumbar area—and a break in the pars evident on radiography. Wiltse
emphasized that some patients may have a “chronic diathesis,” which
predisposes them to injury to the pars with relatively minor trauma,
but this is better termed an isthmic slip
than a true traumatic pars fracture, which requires a great deal of
force. Traumatic olistheses also are seen with bilateral pedicle
fractures and separation of the posterior elements in major trauma.
disease, and osteomalacia, can weaken the posterior elements to the
extent that allows spondylolisthesis. Little has been published on this
condition; most authors group these cases with degenerative
spondylolisthesis.
pars, or one entire facet joint predisposes the spine to instability.
Although this predisposition varies clinically depending on the amount
of preexisting disc degeneration and associated instability, if the
decompression requires sacrifice of these important stabilizing
structures, the addition of an arthrodesis to enhance stability is
required. Instrumentation can be used to augment a posterolateral
fusion.
M, Totty WG, Gilula LA. Spondylolysis of the lumbar spine:
demonstration of defects and laminar fragmentation. Radiology
1984;153:627-629.
DS, Iza J. Repair of the defect in spondylosis or minimal degrees of
spondylolisthesis by segmental wire fixation and bone grafting. Spine
1985;10:673-679.
K. Sedgewick T, O’Brien M, et al. The role of fusion and
instrumentation in the treatment of degenerative spondylolisthesis with
spinal stenosis. J Spinal Disord 1993;6:467-472.
EJ. Single-level posterolateral arthrodesis, with or without posterior
decompression, for the treatment of isthmic spondylolisthesis in
adults: a prospective, randomized study. J Bone Joint Surg
1997;79A:1175-1180.
JS, Mackay M, Herkowitz HN, et al. Degenerative lumbar
spondylolisthesis with spinal stenosis: a prospective, randomized study
comparing decompressive laminectomy and arthrodesis with and without
spinal instrumentation. Spine 1997;22:2807-2812.
BE, Baker D, McHolick WJ, et al. The natural history of spondylolysis
and spondylolisthesis. J Bone Joint Surg 1984;66A: 699-707.
L, Robertson P, Novotny J, Pope M. Etiology of spondylolisthesis:
assessment of the role played by lumbar facet joint morphology. Spine
1993;18:80-92.
HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal
stenosis: a prospective study comparing decompression with
decompression and intertransverse progressive arthrodesis. J Bone Joint
Surg 1991;73A:802-808.
SS, Bradford DS, Tansfeldt EE, Cohen M. Reduction of high-grade
spondylolisthesis using Edwards instrumentation. Spine 1996;21: 367-371.
K, Wilner S, Johnsson K. Postoperative instability after decompression
for lumbar spinal stenosis. Spine 1986;11:107-110.
NH, Lee JW. Anterior interbody fusion versus posterolateral fusion with
transpedicular fixation for isthmic spondylolisthesis in adults: a
comparison of clinical results. Spine 1999;24:812-817.
SM, Connolly PG, Schott S. Degenerative lumbar spondylolisthesis: a
meta-analysis of the literature 1970-1993. Spine 1994;10:2256S-2265S.
H, Hedlund R. Instrumented and non-instrumented posterolateral fusion
in adult spondylolisthesis—a prospective randomized study: II. Spine
2000;25:1716-1721.
FF, Kishore PR, Cunningham ME. Routine oblique radiography of the
pediatric lumbar spine: is it really necessary? AJR Am J Roentgenol
1978;131:297-298.
H. Long-term clinical and radiological follow-up of spondylolysis and
spondylolisthesis. J Pediatr Orthop 1987;7:631-638.
K, Christensen FB, Eiskjaer SP, et al. The effect of pedicle screw
instrumentation on functional outcome and fusion rates in
posterolateral lumbar spinal fusion: a prospective, randomized clinical
study. Spine 1997;22:2813-2822.
