SPINAL STENOSIS
Vice-Chairman, Department of Orthopaedic Surgery, Head, Section of
Spinal Surgery, Cleveland Clinic Foundation, Cleveland, Ohio, 44195.
buttock, or leg pain with characteristic provocative and palliative
features. The term “stenosis” denotes a narrowing or constriction of a
tubular structure. Sachs and Fraenkel (103a)
were among the first to relate symptoms of sciatica to neural
compression within the spinal canal. Subsequent descriptions of this
condition described acquired (degenerative) bony compression and
congenital narrowing of the spinal canal. Van Gelderen (114a)
proposed hypertrophied ligamentum as a potential cause of spinal
stenosis and reported on two patients with this condition. The clinical
features of the syndrome of spinal stenosis and its relationship to
congenital narrowing were described in detail by the Dutch surgeon
Verbiest, who also demonstrated mechanical compression of neural
structures by myelography (116).
Kirkaldy-Willis et al. further defined the pathoanatomy of spinal
stenosis and helped correlate pathologic changes with symptoms (62).
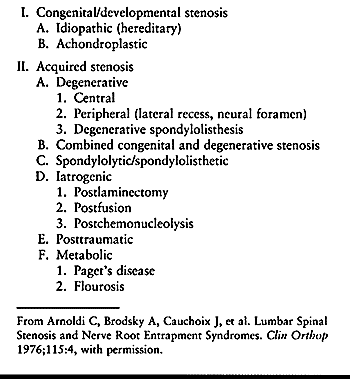
congenital (developmental), acquired (degenerative), or a combination
of both (Table 147.1) (2).
The majority of cases of spinal stenosis are acquired, being caused by
degenerative changes occurring in the three-joint complex consisting of
the intervertebral disc and the two facet joints. In some cases, such
degenerative changes may be
superimposed
on a pre-existing congenital stenosis. Variations in the shape, as well
as the size, of the spinal canal may predispose the patient to spinal
stenosis, with a trefoil canal being associated with lateral recess
stenosis more commonly than a round or oval canal.
 |
|
Table 147.1. Classification of Spinal Stenosis
|
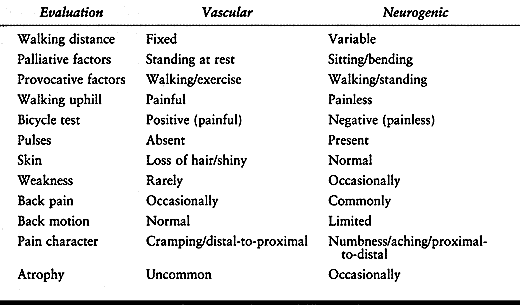
although the two terms are often used interchangeably. Spinal stenosis
refers to morphology, not symptoms. Neurogenic claudication, also known
as pseudoclaudication, is a clinical syndrome with symptoms of leg pain that are associated with walking (116).
Neurogenic claudication should also be distinguished from vascular
claudication, which has a different etiology and slightly different
clinical features (Table 147.2).
 |
|
Table 147.2. Vascular Versus Neurogenic Claudication
|
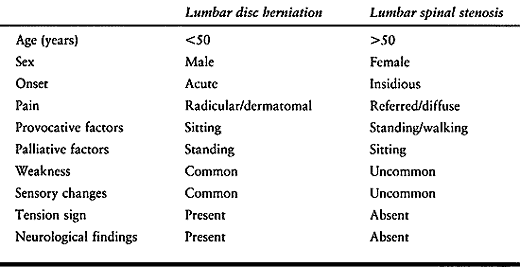
and is typically due to age-related degenerative changes of the lumbar
spine (Table 147.3). The onset of symptoms is
usually insidious and without associated trauma. A history of
antecedent low back pain (LBP) is common, partly because of age-related
spondylosis.
 |
|
Table 147.3. Comparative Features of Lumbar Disc Herniation versus Spinal Stenosis
|
Pain is the predominant symptom, being present in up to 94% of
patients, with numbness (63%) and weakness (43%) being less common.
Bilateral involvement is common. Patients with neurogenic claudication
may present with either unilateral radicular pain or with diffuse,
nondermatomal symptoms beginning in the buttocks and extending a
variable distance into the legs. Radicular pain is typically dermatomal
in distribution and is often unilateral. It is the presenting type of
symptom in 6% to 13% of symptomatic patients. It is often seen with
lateral recess stenosis, foraminal stenosis, or with concomitant
disc
herniation. The presence of a symptomatic disc herniation in a patient
with a narrowed spinal canal and spinal stenosis is not uncommon.
Patients with either developmental or degenerative stenosis are more
likely to develop symptomatic radiculopathy in the presence of a small
disc herniation or even disc bulging (42,94).
Other leg symptoms such as weakness or numbness may also occur in
association with prolonged standing or walking. Night pain is uncommon,
although it has been described in patients with lateral recess stenosis
(54). Unusual symptoms, such as priapism associated with intermittent claudication during walking, have also been reported.
Cadaveric studies have demonstrated that the spinal canal
cross-sectional area, midsagittal diameter, and subarticular sagittal
diameter are significantly reduced in extension (standing) and are
increased with flexion (sitting) (47). Associated neural compression was also found to be greater in extension than in flexion (47).
measurements have shown that epidural pressures at the level of
stenosis were higher in the standing posture compared with those in the
lying and sitting postures. Furthermore, local epidural pressures were
increased with extension and decreased with flexion (47).
Although both conditions may present with leg pain associated with
walking, it is only patients with neurogenic claudication who have leg
pain resulting from standing. Leg pain associated with neurogenic
claudication is highly position dependent. Vascular claudication, on
the other hand, is unaffected by positions of lumbar flexion or
extension. Leg pain from vascular claudication may be produced by
cycling in a sitting position (27). Patients
with vascular claudication typically have leg pain while walking
uphill, whereas patients with a neurogenic etiology do not have this
pain owing to the slightly flexed posture of the lumbar spine
associated with this activity. Patients with neurogenic claudication
may actually have increased leg pain when walking down an incline owing
to increased associated lumbar lordosis.
-
Demographics
-
Typically middle aged or older, unless congenital
-
Female > male (3:1 to 5:1)
-
-
Leg pain > LBP
-
May have long history of antecedent LBP
-
Leg pain
-
Referred or radicular
-
Pseudoclaudication (neurogenic claudication)
-
Provoked by standing or walking
-
Relieved by sitting or leaning (“grocery cart sign”)
-
-
-
Differential diagnosis
-
Peripheral neuropathy (not activity related; burning dysesthesia)
-
Vascular claudication versus neurogenic claudication (Table 147.2)
-
Hip arthropathy (“Hip-spine syndrome”)
-
stenosis by observing the patient, both at rest and during walking.
Because symptoms are typically induced by the normal lordotic posture
associated with walking or standing, the patient often preferentially
assumes a slightly flexed posture in order to relieve neural
compression causing leg pain. Flattening of the lower lumbar spine,
owing to reduction in lumbar lordosis, may also be observed. With
progressive ambulation, the patient may become increasingly more
kyphotic in posture. This represents a conscious, or subconscious,
attempt to decrease root compression by increasing canal or foraminal
size. Back range of motion will likely be reduced as a result of
age-related arthrosis.
Tension signs, such as straight leg raising sign or femoral nerve
stretch test, are uncommon with spinal stenosis unless it is associated
with a disc herniation. Deep tendon reflexes, particularly at the
ankle, may be normal, symmetrically reduced, or absent in the older
patient. Therefore, the presence of diminished reflexes is usually not
clinically significant unless it is asymmetric. Sensory findings, such
as diminution of pinprick sensation, are uncommon with spinal stenosis.
The presence of paresthesias should raise the suspicion of an
underlying peripheral neuropathy.
component. Because of the dynamic nature of spinal stenosis, symptoms
or objective neurologic findings are not usually elicited until these
dynamic factors are invoked. Therefore, resting neurologic examination
is usually normal. The most common neurologic finding is weakness of
the extensor hallucis longus (EHL). Patient symptoms may sometimes be
provoked by either walking or lumbar hyper-extension. Indeed,
reproduction of leg pain by hyperextension of the back may be the only
objective finding (5). Signs and symptoms may
also occasionally be elicited by examining the patient immediately
after walking to the point of producing leg pain. Under such
circumstances, mild muscle weakness or diminution of a tendon reflex
may be detected. Profound muscle weakness is uncommon unless stenosis
is accompanied by concomitant disc herniation. Long tract findings of
spasticity, hyperreflexia, and clonus suggest superimposed cervical or
thoracic myelopathy.
causes for leg pain such as hip arthropathy or peripheral vascular
disease. Include an examination of peripheral pulses and an examination
of the hip. In addition to reproduction of the patient’s pain by hip
range of motion, the presence or absence of a hip flexion contracture
should also be determined because its presence may not only help
explain a patient’s symptoms but also has therapeutic implications.
radiographic findings in order to determine the significance, if any,
of the radiographic finding and the patient’s symptoms. Precise
correlation between objective clinical findings and diagnostic imaging
has been shown to have a high positive predictive value for good
clinical outcome in patients undergoing surgery for symptomatic lumbar
disc herniation. This poses somewhat of a problem in the diagnosis of
lumbar spinal stenosis, in which objective neurologic findings are
usually absent and the clinical diagnosis is made by patient symptoms
rather than clinical findings (53).
evaluation can lead to a poor outcome following surgery, because
radiographic abnormalities, including neural compression, are found in
a significant proportion of asymptomatic individuals (13,45a,50,120).
Unless there is concern for the presence of tumor or infection, avoid
diagnostic imaging when the history or objective clinical findings do
not support a compressive or mechanical cause for the patient’s pain.
Extensive diagnostic imaging can be delayed until the patient is a
clear candidate for surgery.
It has been estimated that only one in 2,500 lumbar radiographs yields
clinically unsuspected findings in patients 20 to 50 years of age.
Numerous studies have reported age-related degenerative x-ray changes
to be present equally in both asymptomatic and symptomatic populations (37). Only the study by Frymoyer et al. (37)
reported a statistically significant correlation between symptoms and
any degenerative finding, that being an association between LBP and
disc space narrowing or traction spurs at the L4–L5 interspace only.
tool. Obtain radiographs in all patients undergoing surgery for spinal
stenosis. Look for unsuspected bony pathology, such as spina bifida
occulta, on plain radiographs of patients undergoing lumbar surgery. In
addition, the presence of transitional vertebrae should be identified
when present, thereby alerting the surgeon to the possibility of errors
in intraoperative localization.
studies for all patients undergoing surgical decompression for spinal
stenosis to identify unrecognized degenerative spondylolisthesis or
degenerative scoliosis, which could be undetectable on supine films.
Preoperative identification of such pathology may influence the type of
the planned surgery, such as the need for concomitant fusion with
decompression. Furthermore, failure to identify a pre-existing
degenerative spondylolisthesis
preoperatively might lead to the erroneous conclusion that a slip seen on a postoperative x-ray study is iatrogenic.
useful than static x-ray studies in making a radiographic diagnosis of
instability (14,41,81). Even with these radiographs, however, there is no uniformly accepted method of measurement of such instability (105).
Shaffer et al. reported that the Morgan and King method of measuring
from the anterior aspect of the vertebral body was the most
reproducible method to measure translation (105). Other authors have described angulation, in addition to translation, as being indicative of radiographic instability (14,41).
normal translation and angulation that can exist in the absence of
symptoms (14,41). Over
90% of asymptomatic volunteers exhibit between 1 and 3 mm of
translation on flexion extension radiographs, and the mean dynamic
sagittal rotation from flexion to extension ranges from 7.7° to 9.4° at
each lumbar level (14). For translation, a
dynamic change of greater than 4 mm is considered abnormal. Because
plain radiographs do not visualize neural structures, they generally
fail to provide an explanation for radicular pain.
evidence of nerve root compression by demonstrating changes in the
contour of normal contrast-filled structures. As such, the exact nature
of compression may be unclear and could, therefore, result in
diagnostic confusion. For example, lateral indentation of the dye
column due to facet arthropathy could easily be confused with that due
to a lateral disc herniation or to a ganglion cyst from a facet joint.
agents. Myelography is superior to routine CT in its ability to image
the entire thoracolumbar spine, thereby revealing unsuspected lesions
at the thoracolumbar junction. This is particularly important with
conditions such as spinal stenosis, in which compressive findings are
often present diffusely throughout the lumbar spine, including the
upper lumbar region, which is not routinely imaged by conventional CT.
superior ability to visualize neural compression associated with
scoliosis afforded by its coronal imaging capabilities. The presence of
a three-dimensional deformity such as scoliosis makes visualization of
neural compression by CT or MRI more difficult than with myelography.
which is located at the level of the pedicle, myelographic dye cannot
extend beyond that point and myelography is unable to detect foraminal
disc herniations, lateral stenosis, or the so-called far out syndrome,
which is diagnosed more accurately by CT or MRI (121).
The far-out syndrome typically occurs in the elderly patient with
degenerative scoliosis or in the younger patient with a grade II or
higher isthmic spondylolisthesis. The L-5 nerve root is compressed far
laterally by either the L-5 transverse process or kinking beneath the
L-5 pedicle.
inability to detect pathology below the level of a complete block to
dye flow (43). This may occur in cases of
severe spinal stenosis, such as with a high-grade L4–L5 degenerative
spondylolisthesis. Under such circumstances, dye must be introduced
both below and above the level of the block, or as is more commonly
done, an adjunctive study such as MRI or CT must be used (43).
MRI is difficult owing to significant metal artifact associated with
the use of stainless steel spinal instrumentation. This problem is
partially obviated by the use of myelography, which is not associated
with image distortion.
asymptomatic patients undergoing oil-based contrast studies for
suspected acoustic neurilemmoma had abnormal lumbar myelography (45a). This finding underscores the importance of correlating radiographic abnormalities with clinical findings.
myelography in the diagnosis of lumbar nerve root compression ranges
from 67% to 100%, depending on the criteria employed for diagnosing
nerve root compression, whether or not surgical confirmation of
compression was used as the standard, and whether or not the tests were
interpreted without knowledge of clinical symptoms or objective
neurologic findings (10,41,48,49,112). Most studies report the diagnostic accuracy of myelography for spinal stenosis to be between 70% and 90%.
and therefore provides more accurate knowledge of the nature of the
compressing lesion. Advantages of CT over myelography include its
noninvasive nature, less ionizing radiation, and a better ability to
visualize lateral pathology such as lateral or foraminal disc
herniation or foraminal stenosis. Because CT is usually performed
without sagittal reformation, it provides imaging in only one plane and
routinely images only a limited segment of the spine. Therefore, CT
misses proximal lumbar pathology, such as a high lumbar disc
herniation, proximal stenosis, or other significant pathology (e.g., a
thoracolumbar
tumor) unless it is specifically oriented to those levels. Because
spinal stenosis is a global condition, commonly involving upper lumbar
segmental levels as well as lower lumbar levels, routine use of only CT
as the primary imaging tool would result in some missed diagnoses.
reported that 35.4% of asymptomatic individuals in their study group
had an abnormal CT scan. Reported accuracy of CT in the diagnosis of
nerve root compression from disc herniation or stenosis ranges from 72%
to 100% (10,48,49,112).
use of water-soluble contrast agents (intrathecal contrast-enhanced CT
or myelo-CT). The incremental benefit provided by combining both
procedures is so great that they are usually performed sequentially as
part of a single study for spinal stenosis. Postcontrast CT allows
distinction between the disc margin, thecal sac, and ligamentum flavum,
three structures that can blend together in a tight spinal canal in
which normal tissue-separating fat is absent. It is invaluable in
visualizing a stenotic spine associated with a complete myelographic
block, as in severe lumbar stenosis associated with degenerative
spondylolisthesis (43). Correlation between
contrast-enhanced CT and myelography ranges between 75% and 96%, with
myelo-CT invariably being the more accurate study (43,48,49,112).
been assigned a shade of gray based on the intensity of a radio wave
signal emanating from the tissue (7,8).
In the lumbar spine, T1-weighted sagittal and axial sequences of
approximately 4 mm slice thickness and sagittal gradient echo (GE)
sequences are performed most commonly. Typically, osseous structures
appear as areas of relative signal void, with cortical bone having a
low intensity on MRI, and cancellous bone having a higher signal
intensity owing to its fat content. The distinction between a small
cortical bone osteophyte and a small disc herniation on T1-weighted
sagittal image may be difficult, and precise differentiation between
the two features may require CT. The nucleus pulposus is best
visualized by T2-weighted spin echo (SE) sequences, which reflect the
degree of hydration of the disc. With aging and disease, there is
decreased signal intensity due to changes in total hydration within the
disc (13). The T2 image tends to overemphasize
the size of a disc herniation and, therefore, can overestimate its
potential significance.
can detect unsuspected pathology such as high-lumbar disc herniation,
proximal stenosis, or thoracolumbar spinal tumor. MRI is noninvasive
and eliminates the potential risk and associated discomfort associated
with myelography. Like CT, MRI visualizes the spine directly,
providing detail as to the etiology of neural compression and can
accurately image lateral pathology. Unlike routine CT, however, MRI
provides sagittal visualization of the spine and, therefore, provides
imaging in orthogonal planes. Furthermore, MRI uses parasagittal views,
which provide sequential visualization of neural foramina and can
detect foraminal entrapment better than routine CT. This feature is
particularly valuable for imaging spinal stenosis, in which neural
entrapment within or beyond the neural foramen can be well visualized.
MRI distinguishes between the disc and neural tissue better than
nonenhanced CT but generally does not distinguish between bony and
soft-tissue compression as well as CT. When this distinction is deemed
important, as it sometimes is in cases of spinal stenosis, CT or
contrast-enhanced CT is sometimes needed.
are common in asymptomatic individuals. In one study of asymptomatic
subjects, the lumbar MRI images of 22% of those younger than age 60 and
57% of those older than age 60 were abnormal, showing disc herniation
or spinal stenosis (13). Approximately 90% of
those older than 80 years of age showed some element of lumbar disc
degeneration, as demonstrated by decreased signal on T2-weighted
images. The reported accuracy of MRI, when compared with documented
intraoperative lumbar nerve root compression, is comparable to that of
contrast-enhanced CT (myelo-CT) (9,49).
because good studies documenting the course of nontreatment are
lacking. This is partly because most patients with this condition
receive some form of conservative or surgical treatment, and those with
severe stenosis are ultimately operated on (12).
Several reported studies have described the clinical features of spinal
stenosis, or its surgical treatment, and have included some patients
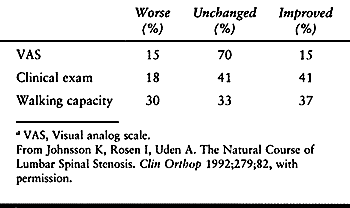
who received no treatment (51). Approximately 20% of those receiving no treatment experienced progression of their symptoms.
who reported on 32 patients with spinal stenosis followed for an
average of 49 months. These patients were described as having
“conservative treatment (i.e., no treatment)” because either the
patient refused to undergo surgery or the anesthesiologist refused to
administer anesthesia. Therefore, these patients had indications for
surgery but were not operated on. At final follow-up, based on the
clinical examination, 41% of patients were improved and 18% were worse.
Based on subjective symptoms, only 15% were improved and 15% were
worse. Changes in the patients’ walking capacities were equally distributed among improved, worse, and unchanged (Table 147.4).
When the final outcome was compared with the anteroposterior (AP)
diameter of the dural sac, as measured on water-soluble contrast
myelography, patients with narrow AP diameters had a tendency not to
improve. This study concluded that the majority of patients with spinal
stenosis who did not undergo surgery remained unchanged at 4 years of
follow-up and severe progression was unlikely.
 |
|
Table 147.4. Final Outcome for Untreated Spinal Stenosis by VASa, Clinical Exam, and Walking Capacity
|
the outcome of a group of 44 patients treated with surgical
decompression with that of 19 patients treated without surgery (Table 147.5). The authors referred to the nonsurgical group as both untreated and conservatively treated,
leaving unanswered what, if any, treatment this group did receive.
Nevertheless, the authors found that only 32% of the nonsurgical
patients had improved at an average follow-up of 31 months. In the surgical
group, 59% of the patients reported improvement. Although 59% of the
group who had received surgery improved, a greater percentage of the
surgical group were worse at follow-up compared with patients who had
not undergone surgery (25% versus 10%). This study concluded that
nonsurgical treatment produced reasonably good results in approximately
one third of patients, with only a 10% chance of deterioration during
the 2- to 3-year follow-up period. This study, however, was not
prospective nor randomized, making comparison between the two groups
difficult. In the absence of randomization, it is not known whether the
conservatively treated patients were comparable to the surgical group.
 |
|
Table 147.5. Comparison of Surgical Versus Nonsurgical Treatment of Lumbar Spinal Stenosis
|
surgery for spinal stenosis failed to identify a single randomized
trial comparing surgery with conservative treatment (113).
A recent report evaluating the outcome of patients treated with
aggressive nonsurgical measures (therapeutic exercises and epidural
steroids, if necessary) suggested that such treatment could be very
effective (103). Fifty-two patients were
followed for 2 to 8 years. Thirty-three patients (63%) reported a
tolerable pain level without major restriction in daily activities or
use of narcotic analgesics; 36 patients (69%) reported “no or minimal
restriction in walking tolerance,” although 25 patients (48%) reported
“difficulty in standing for long periods.” None of the patients
experienced any neurologic loss. Four of the 52 patients (8%) required
surgery for presumed failure of nonsurgical measures. The exclusion
criteria for this study included patients with pre-existing disease
(comorbid conditions) or with a “compliance issue that prevented
participation in a therapeutic exercise program.” In addition, it did
not compare conservative treatment methods with surgery and could not,
therefore, offer any comparative data regarding optimal treatment of
this condition.
stenosis include nonsteroidal anti-inflammatory medications,
analgesics, oral and epidural steroids, physical therapy, bracing, and
calcitonin (109).
divided into decompressive procedures without concomitant fusion and
decompression with fusion. Surgical decompression may vary from limited
procedures, such as single-level unilateral laminotomy for focal neural
compression, to global procedures, such as multilevel bilateral
laminectomy with bilateral facetectomies. Types of fusion procedures
include anterior lumbar interbody fusion (ALIF), posterior lumbar
interbody fusion (PLIF), posterior fusion, posterolateral (also known
as intertransverse or bilateral lateral) fusion, or combinations of
these procedures
(see Chapter 145 and Chapter 146).
Indirect neural decompression may occur following ALIF or PLIF if
disc-space distraction occurs, thereby enlarging the central or
foraminal canal. Fusion may be augmented by the use of spinal
instrumentation, either anterior fixation devices or posterior devices
such as those using pedicle screw fixation.
The relationship between comorbidity and outcome is more commonly
applied to surgical than medical outcomes. Comorbidity typically
increases with age and is associated with a poor outcome for many
medical and surgical conditions (20,25,107).
Sick people have a higher mortality rate, a higher complication rate,
and a lower level of function than do healthy patients. It is
imperative to take this factor into account when assessing and
comparing outcomes between treatment groups. If such factors are not
taken into account, differences in outcome between treatment groups
could reflect differences in patient comorbidities rather than
differences as a result of treatment.
Complications are more frequent with advancing patient age, increasing
the complexity of both diagnosis and surgical treatment. The study by
Deyo et al. (25) reported an overall mortality
of 0.07% for 18,122 hospitalizations between 1986 through 1988. The
mortality increased with age, increasing to 0.6% (ninefold increase) in
patients older than 75 years of age. The overall complication rate of
9.1% increased to 17.7% in patients 75 years of age or older.
often associated with more in-hospital complications and perioperative
mortality. This finding is independent of age alone. Oldridge et al. (86)
found an age-related increase in mortality only for patients older than
80 years of age. There was, however, a significant increase in
in-hospital and 1-year cumulative mortality associated with increasing
number of comorbidities.
By 1 year after surgery, 6% of patients had a second operation and, by
the time of the last follow-up, 17% had a repeat surgery. Only 40% of
patients with the highest comorbidity score had a good outcome at the
time of final follow-up compared with 75% of patients who had the
lowest comorbidity score (P = 0.004). The most common comorbidities
were osteoarthritis (32%), cardiac disease (22%), rheumatoid arthritis
(10%), and chronic pulmonary disease (7%). Their data suggested that
the effect of comorbidities was additive, because no single comorbidity
was significantly associated with worse outcome. In a subsequent study
by the same authors, comorbidity was found to be the second most
important determinant of disability in lumbar canal stenosis, with
complaints of predominantly LBP (as opposed to leg pain) preoperatively
being the most important contributor to disability (55,58).
 |
|
Table 147.6. Long-Term Outcome Following Surgery for Spinal Stenosis
|
For bilateral laminectomy, the lamina and ligamentum flavum are removed
on both sides of the stenotic level or levels to the lateral recess.
Decompression begins at the most distal extent of neural compression
and proceeds in a caudal-to-cranial direction. Although the L5–S1 level
is rarely compressed centrally, owing to the capacity of the spinal
canal at that level, decompression is most safely initiated at that
level rather than at L4–L5, the most commonly involved level, which is
often severely stenotic. Perform decompression sequentially, from
medial to lateral.
-
Position the patient in a kneeling
position to allow the abdomen to hang freely in order to reduce
abdominal compression and thereby reduce epidural bleeding. Prepare and
drape the lumbosacral spine and expose the posterior elements from
facet joint to facet joint laterally along the entire length of the
intended decompression. -
Begin with a midline decompression. This
is generally performed from the left side of the operating table (i.e.,
on the patient’s left side) for a right-handed surgeon and on the right
side by a left-handed surgeon. -
Use either 45° or 90° Kerrison rongeurs.
In areas where stenosis is not severe, use a relatively large rongeur,
such as a 4 mm Kerrison rongeur, to remove the thickened lamina. -
In areas of severe stenosis, however, use
of such large instruments risks injury to underlying neural structures.
Under these circumstances, it is safer to first thin the lamina with
either a Lexsell rongeur or a high-speed power burr. Then use smaller
instruments, such as 2 mm or 3 mm Kerrison ronguers, to complete the
midline decompression. -
Maintain proper orientation during the
procedure by identifying the level of the pedicle, because this defines
the level of the nerve root. If in doubt as to the proper level,
confirm with an intraoperative radiograph with a bent probe beneath the
pedicle, within the neural foramen. -
Decompress the lateral recess next.
Extend the decompression laterally until the lateral edge of the root
is visualized and determined to be free of pressure. Take care to
preserve the pars interarticularis to minimize the risk of producing
instability by inadvertent sacrifice of the superior articular facet.
Preserve the facet joint by using oblique-angled (45°) Kerrison
rongeurs or by the use of osteotomes to undercut the facet joint (39,104). -
Finally, perform lateral decompression of
the foraminae. Once the shoulder of the nerve root is identified and
decompressed, follow it from its origin through the neural foramen. -
It is generally safer to proceed in a
cranial-to-caudal manner in order to minimize risk of inadvertently
cutting across the root, which can occur when performing the lateral
decompression from a distal-to-proximal direction. Occasionally, the
use of a right- or left-angled Kerrison rongeur can be helpful for
foraminal decompression. -
Assess the adequacy of decompression
within the neural foramen both visually and by palpation. Use a bent
probe, such as a bent #4 Penfield elevator or a properly contoured ball
probe, to determine the presence or absence of nerve root compression
within the neural foramen. Decompression is generally complete when a
bent probe can be passed out the foramen both dorsal and ventral to the
nerve root, and the root can be gently retracted approximately 1 cm
medially.
the presence or absence of a concomitant disc herniation, which might
contribute to neural compression. Such herniations may be located
either posterolaterally, foraminally, or extraforaminally. Unless the
disc is contributing to definite neural compression, it is generally
best to avoid discectomy in the presence of laminectomy because
subsequent instability is more likely to occur when both anterior and
posterior supporting structures are violated. When laminectomy is
accompanied by discectomy, consider performing an arthrodesis at the
time of surgery.
process, encompassing multiple levels and involving nerve roots
bilaterally, multilevel bilateral laminectomy is commonly required.
There is, however, some debate as to whether it is more appropriate to
decompress only the symptomatic level and side, or whether all stenotic
levels should be decompressed. The argument against decompression of
asymptomatic root levels or sides is the risk of producing symptoms at
a previously asymptomatic level or side. On the other hand, failure to
decompress a stenotic but asymptomatic level or side risks progression
of the degenerative process with the development of more severe and
potentially symptomatic stenosis. In addition, the natural tendency for
degenerative changes to progress over time makes it possible that, in
time, asymptomatic stenotic levels will eventually become stenotic.
Indeed, several studies have reported long-term deterioration following
initially successful surgical decompression (18,19,56,60,90,91 and 92).
unilateral, rather than bilateral, removal of bone and ligamentum
flavum. Because the spinous processes, interspinous ligaments, and
supraspinous ligaments are preserved medially, normal stabilizing
structures are retained with less risk of development of postoperative
instability. Take care to preserve the pars interarticularis laterally
in order to minimize risk of postoperative instability (16,106).
Hemilaminectomy is appropriate for patients with unilateral symptoms
from stenosis. A disadvantage of this procedure is the relative
difficulty of performing contralateral decompression and also in
obtaining enough medial exposure to perform an adequate ipsilateral
decompression in patients with foraminal stenosis. The presence of an
intact spinous process and interspinous or supraspinous ligament
complex makes it difficult to angle the Kerrison rongeur laterally
enough to insert the jaw of the rongeur into the depths of the neural
foramen. Under such circumstances, removal of the midline spinous
process and interspinous or supraspinous ligament complex may be
necessary in order to allow the proper angulation of the rongeur to
perform the foraminal decompression. In addition to preserving midline
stabilizing structures, hemilaminectomy also avoids exposure of, and
potential injury to, the contralateral facet joint. Because the
integrity of the unexposed contralateral facet is maintained, more
aggressive decompression of a nerve root by partial, or even total,
ipsilateral
facetectomy need not necessarily be accompanied by a fusion.
accomplished through a unilateral hemilaminectomy approach by tilting
the table away from the operating surgeon (Fig. 147.1).
Particularly when used in conjunction with an operating microscope,
which provides excellent illumination and which can be angled to
visualize the opposite side, contralateral decompression can be
accomplished without the need for removal of stabilizing midline
structures (spinous processes and interspinous or supraspinous
ligaments). The contralateral neural foramen can be visualized and
decompressed, and its more distal portion can be palpated with a long
bent probe such as a #4 Penfield elevator or a contoured probe.
Although offering the advantage of preserving normal, noncompressing
midline structures and minimizing scar tissue on the opposite side,
this technique is more demanding than bilateral laminectomy because
decompression is performed through a more limited exposure and the
determination of adequate foraminal patency is more dependent on feel
(palpation) than by direct visualization. In addition, there is a
greater potential for dural laceration from the Kerrison rongeur when
working through a small opening. Should such a dural tear occur, its
repair often necessitates complete (bilateral) laminectomy with
adequate exposure of the dural rent.
 |
|
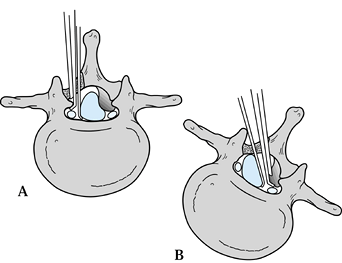
Figure 147.1. A:
Axial representation of hemilaminotomy showing ipsilateral decompression of the nerve root. The operating table can be tilted toward the surgeon to facilitate visualization of the contralateral spinal canal. A Kerrison rongeur is shown decompressing the nerve root within the lateral recess, while a Penfield retractor is protecting the common dural sac medially. B: Axial representation of hemilaminotomy showing contralateral decompression of nerve root. The operating table is tilted away from the surgeon. The Kerrison rongeur is shown decompressing the opposite nerve root, while the Penfield retractor is gently moving the common dural sac medially to facilitate visualization of the contralateral nerve root. |
surgery failed to identify even a single randomized trial comparing
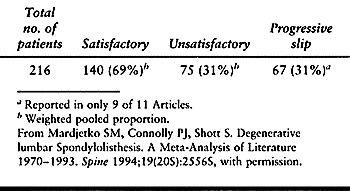
surgery and conservative treatment (Table 147.7) (113). Turner et al. (113)
attempted a meta-analysis of the literature on surgical outcomes for
spinal stenosis, but the poor scientific quality of the literature
precluded the authors from conducting the intended meta-analysis. Even
using the authors’ own ratings, the average proportion of
good-to-excellent outcomes was only 72%. This study found no
statistically significant relationship between outcome and patient age,
gender, presence of prior back surgery or number of levels operated on.
In those studies reporting on only patients with degenerative
spondylolisthesis, the outcome was better. There was no statistically
significant difference in outcome between decompression with or without
associated fusion. This observation is particularly significant in
light of the reported increased morbidity associated with lumbar fusion
(114).
 |
|
Table 147.7. Results of Decompression of Spinal Stenosis without Fusion: Meta-Analysis of Literature 1970–1993 (11 Articles)
|
reported the 1-year outcome of patients with spinal stenosis treated
surgically or nonsurgically in the state of Maine and found that at 1
year, 55% of the surgical patients reported definite improvement in
their predominant symptom, compared with only 28% of the nonsurgical
group. Surgery was found to increase the relative odds of “definite
improvement” 2.6 fold compared with nonsurgical treatment.
Outcome assessment included a questionnaire in which the patients rated
their outcomes in terms of pain and function. The authors reported a
surprisingly high failure rate, with 11% of patients reporting a poor
outcome at 1 year and 43% reporting poor outcome at final follow-up.
Six percent of patients had repeat lumbar surgery
within
the first year and 17% had additional surgery by the time of last
follow-up. The authors concluded that the long-term outlook for
patients undergoing decompressive laminectomy for spinal stenosis is
guarded owing to progressive deterioration of the results over time.
They suggested that more extensive bone removal may be indicated at the
time of initial surgery.
following surgery, but by 5 years, the failure rate had reached 27%,
with a predicted failure rate of 50% within the anticipated life
expectancy of most patients. More than half (62%) of these failures
were due to subsequent neurologic symptoms, with an equal incidence of
recurrent stenosis at the same level and stenosis at a new level.
Because of the high rate of failure from recurrent stenosis, the
authors recommended that all levels of impending stenosis be
decompressed along with the symptomatic levels.
of standard bilateral decompressive laminectomy over time, more limited
alternatives to decompressive laminectomy and hemilaminectomy have been
espoused in order to avoid removal of normal, noncompressing structures
and thereby minimize risk of postoperative instability (18,54,60,91). Such procedures include hemilaminotomy, wide fenestration, and laminoplasty. Hemilaminotomy involves a more limited decompression than hemilaminectomy (Fig. 147.2).
Rather than removing an entire hemilamina, hemilaminotomy removes only
the ligamentum flavum and adjacent portions of two hemilaminae
responsible for neural compression. This procedure is more commonly
performed in younger patients with unilateral focal stenosis in whom
extensive laminectomy carries the risk of instability. It may also be
considered in older patients who do not have extensive global stenosis.
 |
|
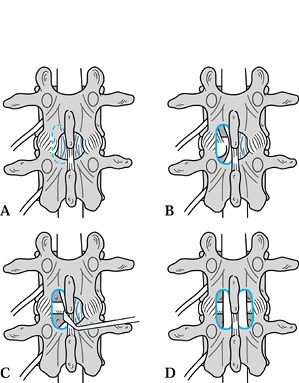
Figure 147.2. Posterior view of a hemilaminotomy to decompress nerve root. A:
Dotted line on left represents the inferior portion of the superior lamina, which is resected in order to decompress the dural sac. This allows identification of the origin of the ligamentum flavum, which attaches approximately half way up the deep surface of the lamina. B: Diagram showing the resected distal portion of the superior lamina and ligamentum flavum to reveal the underlying dura. The common dural sac is deviated medially by an underlying disc herniation. C: The common dural sac is gently retracted medially to facilitate lateral decompression of the facet joint or disc. D: Diagram showing bilateral hemilaminotomies with preservation of midline laminae and ligamentous complex. |
-
In the absence of significant underlying
congenital stenosis, neural compression is generally due to buckling of
the ligamentum flavum, which is usually secondary to collapse of the
intervertebral disc, and to hypertrophy of the facet joint, which
occurs as a result of subsequent instability. Decompression of only
these structures should, therefore, relieve symptoms of neural
compression. -
Because the superior attachment of the
ligamentum flavum is approximately at the midpoint of the deep surface
of the superior hemilamina, resect the distal half of the superior
hemilamina in order to remove the proximal extent of the ligamentum (Fig. 147.2A). -
Remove the inferior portion of the
superior hemilamina and the superior portion of the inferior
hemilamina, together with the intervening ligamentum flavum (Fig. 147.2B). -
Perform lateral decompression by partial facetectomy as with bilateral laminectomy or hemilaminectomy (Fig. 147.2C).
-
Like hemilaminectomy, contralateral
decompression with preservation of spinous processes and midline
supraspinous or interspinous ligaments can be performed by tilting the
operating table away from the surgeon and by undercutting the medial
and contralateral ligamentum flavum with a 45° Kerrison ronguer.
procedure described for central stenosis in which only the medial
portion of the inferior facets and adjacent ligamentum flavum is
removed (69,83,123).
Care is taken to remove only pathologic anatomy and to preserve the
interspinous or supraspinous ligament complex and spinous processes,
which make up the midline stabilizing structures. This may be performed
by using bilateral laminotomies at one or more segmental levels,
removing the ligamentum flavum (Fig. 147.2D). In a 5-year follow-up study of this procedure, 82% of patients had good or excellent early surgical outcomes, but results
deteriorated to 71% satisfactory by 4 years postoperatively (83).
This procedure is similar to cervical laminoplasty and involves hinging
open the lamina on one side and inserting the excised spinous processes
into the open hinge in order to keep it patent. There is not sufficient
experience with this technique to provide outcomes assessment.
is somewhat controversial. For stenosis not associated with
degenerative spondylolisthesis or other deformity, most studies report
that simple decompression is the preferred method of surgical
treatment. For patients with associated degenerative spondylolisthesis,
concomitant fusion is generally recommended (30,44). The issue of using supplementary spinal instrumentation is yet unresolved (32,125).
undergoing either decompression alone or decompression with fusion for
spinal stenosis without associated instability, there was no
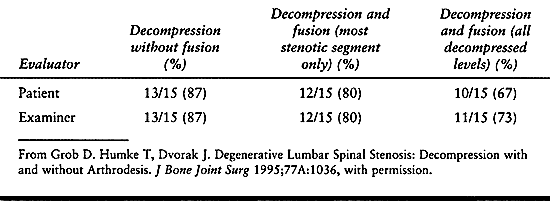
significant difference in outcome between fused and unfused groups (Table 147.8) (40).
Overall, 78% of patient-reported and 80% of examiner-rated results were
rated very good or good. When broken down by type of procedure
performed, there were no significant differences in outcome between the
three groups with regard to pain relief. The authors concluded that
surgical decompression changed the natural history of spinal stenosis,
resulting in generally favorable outcome and improved quality of life
in the majority of patients. They further concluded that arthrodesis
was not justified in the absence of radiographically proven segmental
instability because there was no statistical difference in outcome
between the three treatment groups.
 |
|
Table
147.8. Comparison of Decompression, Decompression and Fusion, and Limited Decompression and Fusion in Spinal Stenosis: Percentage of Good to Excellent Results |
The clinical and pathologic features of this entity were further
defined by Macnab, who described the condition as “spondylolisthesis
with an intact neural arch” (70). The term degenerative spondylolisthesis
was originally used by Newman and Stone and is the terminology most
commonly used to describe the anterior slippage of one vertebral body
on another in the presence of an intact neural arch (84a).
LBP and leg pain and may contribute to radicular or referred leg pain
in a characteristic pattern of neurogenic claudication (36).
The diagnosis is typically made on lateral radiographs, but it may have
a dynamic component to it such that the slip may reduce in the supine
position and, therefore, may be readily apparent only on stress
radiographs. Such radiographs may include standing lateral views,
sitting or standing flexion-extension views, or distraction compression
radiography (14,41).
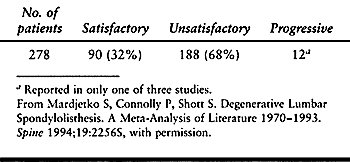
attempted a meta-analysis of the literature from 1970 to 1993. Only
three papers, reporting on 278 patients, described the natural history
of degenerative spondylolisthesis (33,78,96). Overall, 90 of these 278 patients (32%) achieved satisfactory results untreated (Table 147.9).
Matsunaga et al. (78)
presented a study of 40 patients who received no treatment and who were
followed for at least 5 years (range: 5 to 14 years.; mean: 8.25 year).
Progressive slip was noted in 12 patients (30%), although no
correlation was noted between slip progression and worsening of
symptoms. Only 4 of 40 patients (10%) showed clinical deterioration
over the course of the study, all of whom were in the group of 28
patients showing no slip progression over the follow-up period.
Interestingly, none of the 12 patients with slip progression
deteriorated clinically. Therefore, the majority of the patients in
this study showed a slight improvement in their clinical symptoms over
time, although only 1/3 were felt to have satisfactory function at
final follow-up.
 |
|
Table 147.9. Non-Operative/Natural History of Degenerative Spondylolisthesis (Three Studies Reviewed)
|
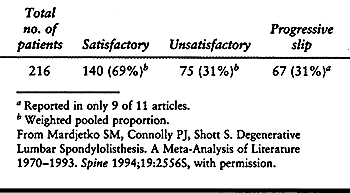
fusion for spinal stenosis associated with degenerative
spondylolisthesis, decompression without fusion is also a viable
therapeutic option (Table 147.10) (74).
Overall, 69% of patients from Mardjetko’s meta-analysis reported
satisfactory outcome with decompression without fusion, with 31% having
an unsatisfactory result and 31% having progression of their slip.
There was generally no correlation between clinical outcome and amount
of slip progression except in the study by Bridwell et al., which
showed a positive correlation between the two (15).
 |
|
Table 147.10. Results of Decompression without Fusion: Meta-Analysis of Literature 1970–1993 (11 Articles)
|
reviewed 290 patients undergoing decompression without fusion for
degenerative spondylolisthesis. Only patients with a “stable” slip, as
defined by a slip having less than 4 mm translation and less than 10°
to 12° angulation on dynamic lateral radiographs, were included. Two
hundred and fifty patients had one-level listhesis and 40 had a
two-level slip. Decompressive procedures included laminectomy in 249
patients and fenestration procedures in 41 patients. Fenestration
procedures typically involved bilateral laminotomy with partial medial
facetectomy and foraminotomy. At an average 10-year follow-up (range: 1
to 27 years), 69% of patients exhibited excellent, 13% good, 12% fair,
and 6% poor outcome. The authors concluded that 82% excellent or good
outcome was very acceptable in their elderly population (average age:
67 years old), in whom fusion is associated with higher morbidity and
mortality (26).
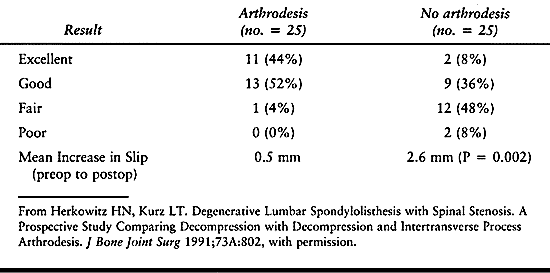
fusion was reported in the prospective randomized study by Herkowitz
and Kurz (44) comparing decompression alone with combined decompression and noninstrumented fusion (Table 147.11).
In the decompression group, only 11 of 25 patients (44%) had a
satisfactory result. This group of patients was found to have
significantly more LBP and leg pain than their fused counterparts.
Furthermore, the mean slip increased from an average of 5.3 mm
preoperatively to 7.9 mm postoperatively. Other authors have reported a
similar experience (15,30).
 |
|
Table
147.11. Prospective, Randomized Comparison of Decompression versus Decompression and Noninstrumented Spinal Fusion for Degenerative Spondylolisthesis |
stenosis associated with degenerative spondylolisthesis is less
controversial than the role of fusion in the treatment of other
degenerative back conditions (80,115). Caputy and Luessenhop (18)
reported a retrospective review of 96 patients undergoing decompressive
surgery for spinal stenosis who were followed for at least 5 years. The
treatment failed in 16 patients because of recurrent neural
involvement, and it failed in 10 patients because of LBP (total
failures = 26). The authors concluded that because of the higher
incidence of recurrent symptoms in patients with pre-existing
degenerative spondylolisthesis, all patients with an associated slip
should undergo fusion of the listhetic level.
decompression alone with decompression and noninstrumented spinal
fusion in the treatment of degenerative spondylolisthesis with spinal
stenosis, Herkowitz and Kurz (44) reported superior results when concomitant fusion was performed with the decompression (Table 147.11).
The reported outcome for the arthrodesis group was excellent in 44% and
good in 52% (96% excellent or good total results), whereas in the
nonarthrodesis group, only 8% reported an excellent outcome and 36%
reported a good outcome (44% excellent or good total results) (P =
0.0001). There was a significant increase in the preoperative slip in
patients not receiving an arthrodesis compared with those undergoing
fusion (P = 0.002). Interestingly, 36% of those undergoing attempted
arthrodesis were noted to have a pseudarthrosis, all of whom had either
an excellent or a good result. This study concluded that the results of
surgical decompression with in situ
arthrodesis are superior to those of decompression alone. The authors
further concluded that the decision for concomitant arthrodesis should
be based purely on the presence or absence of a preoperative slip
rather than on other preoperative factors, such as the age or sex of
the patient or the disc height, or on intraoperative factors such as
the amount of bone resected during the decompression.
included a subgroup of 11 patients undergoing decompression and
noninstrumented fusion. Of the 10 patients available for follow-up,
only 3 (30%) reported improved functional outcome and seven had an
increase in their preoperative spondylolisthesis.
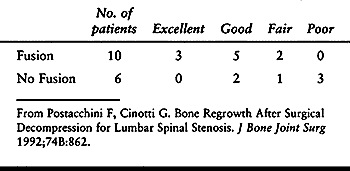
Although all 16 patients with degenerative spondylolisthesis showed
some bone regrowth, the degree of regrowth was more severe in the six
patients who did not undergo arthrodesis. Furthermore, the proportion
of satisfactory results was significantly higher in patients who had
spinal fusion (Table 147.12). Although this
study was nonrandomized and retrospective, it suggested that
arthrodesis stabilizes the spine, resulting in less bone regrowth and
superior long-term results.
 |
|
Table 147.12. Relationship Between Outcome and Fusion in Patients with Degenerative Spondylolisthesis
|
noninstrumented fusion for a variety of diagnoses. The overall fusion
rate for the noninstrumented group was 65%; for the semirigid fixation
group, the fusion rate was 77%; and for the rigid fixation group, it
was 95%. A trend for better clinical outcome with increasing rigidity
of fixation was also observed. Seventy-one percent of the
noninstrumented patients, 89% of the semirigid group, and 95% of the
rigid group reported excellent or good results. For the subgroup of
patients with degenerative spondylolisthesis, 65% of the
noninstrumented patients fused compared with 50% of the semirigid
fixation group, and 86% of the rigid fixation group had a good or
excellent result. Subsequent studies have also reported superior
results with concomitant arthrodesis and decompression for spinal
stenosis with degenerative spondylolisthesis (18,44,60,90).
involved a retrospective, multicenter study of 2,684 patients with
degenerative spondylolisthesis. Solid radiographic fusion was noted in
89% of patients undergoing pedicle screw fixation compared with 70% of
those without instrumentation. Clinical outcome was also better in the
group of patients undergoing instrumented fusion.
retrospective study of 30 patients undergoing decompression and
instrumented fusion for degenerative spondylolisthesis. Outcome was
determined by fusion rate, a functional questionnaire, and the SF-36
survey. Both the rate of fusion and patient satisfaction was 93%.
Thirteen patients (43%) had complications, including dural tears (three
patients), excessive blood loss (two patients), pseudarthrosis (two
patients), pulmonary embolus (PE) (one patient), deep infection (one
patient), urinary tract infections (3 patients), and unstable angina
(one patient).
lumbar fusion, with and without pedicle screw instrumentation, for a
variety of conditions concluded that the addition of instrumentation
did not produce an incremental clinical benefit to that obtained from
noninstrumented fusion, although there was a slight nonsignificant
trend toward a higher fusion rate in the instrumented fusion group (34).
This study, involving a mean clinical follow-up of 40 months,
prospectively examined 71 patients undergoing posterolateral fusion for
either failed back surgery syndrome (FBSS), degenerative disc disease,
isthmic spondylolisthesis, or degenerative spondylolisthesis. For the
10 patients who had degenerative spondylolisthesis, five underwent
instrumented fusion and five underwent fusion in situ.
Eighty percent of the patients with degenerative spondylolisthesis
undergoing instrumented fusion achieved an excellent or good outcome,
compared with 40% of those without instrumentation. For the small
subgroup of 10 patients with degenerative spondylolisthesis, the
clinical outcome appeared to be better than that of the overall
population studied, although this subgroup was too small to establish
statistical significance.
optimal way to treat the patient with degenerative spondylolisthesis.
Most studies suggest that patients undergoing concomitant fusion do
better when decompression is accompanied by fusion (44). It is less clear, however, whether or not the fusion should be augmented with instrumentation (32).
It would seem reasonable that if there is clear evidence of instability
on flexion-extension radiographs, the immediate stability provided by
instrumentation would warrant the additional time, expense, and
potential morbidity associated with its use. On the other hand, the
indication for its use in the patient with a collapsed disc space and
no motion at the spondylolisthetic level is less clear.
spinal stenosis, particularly when it is associated with degenerative
spondylolisthesis, is still somewhat controversial. One area of
controversy is the recommended extent of surgical decompression.
Because spinal stenosis is a global
degenerative condition, there are frequently many segmental levels
showing radiographic central stenosis, with bilateral foraminal
stenosis also being common. Clearly, decompression of every level
showing any degree of radiographic stenosis is not always required.
Obviously, all symptomatic levels should be decompressed. The extent of
surgical decompression of asymptomatic levels, however, depends on many
factors. As described earlier, many long-term studies suggest that
restenosis at previously decompressed levels, or the development of
symptomatic stenosis at previously nonoperated stenotic levels, is a
common reason for failure of surgery for spinal stenosis. Therefore,
when in doubt, it is generally more prudent to decompress a suspicious
segmental level than not to decompress. I generally decompress all
moderately and severely stenotic levels. When diffuse degenerative
changes produce moderate or severe multilevel stenosis, I prefer to
decompress the involved levels by unilateral or bilateral laminotomies,
rather than by complete laminectomies. This approach reduces the need
for concomitant fusion, and it preserves the uninvolved laminae and
ligamentous structures, thereby and minimizing the risk of developing
late instability.
the surgeon whether or not to decompress an adjacent level above or
below the operated level. When this occurs, the degree of central
stenosis of the adjacent segment can be gauged by passing a small
catheter proximally or distally
beneath the lamina. Difficult passage of the catheter mandates decompression of the involved level.
patient with stenosis associated with degenerative spondylolisthesis
can be difficult in the elderly patient with multiple comorbidities. As
noted previously, many studies suggest that patients have better
clinical outcomes when decompression is accompanied by arthrodesis. The
issue of whether or not to augment the fusion with segmental (pedicle)
instrumentation is not yet resolved (32,34,125). The decision to fuse must be balanced against the increased morbidity associated with arthrodesis in the elderly patient (26).
In the younger, healthy patient with spinal stenosis associated with
degenerative spondylolisthesis, I will generally fuse the listhetic
level, usually with segmental fixation. In elderly, debilitated, or
low-demand patients, arthrodesis may not be required. This is
particularly true when the listhetic level is associated with decreased
disc height, spur formation, subchondral sclerosis, or ligament
ossification. These degenerative changes may help stabilize the
listhetic level and minimize the risk of slip progression. Under such
conditions, I consider unilateral or bilateral laminotomies in order to
preserve uninvolved stabilizing structures.
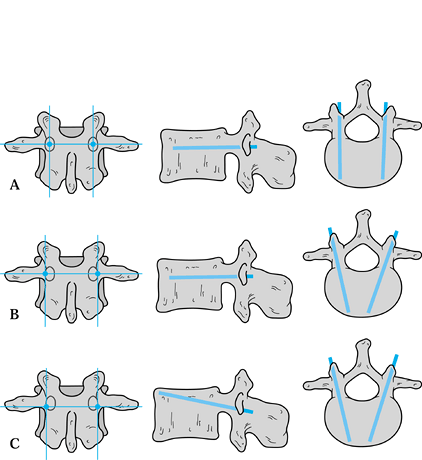
of a line bisecting the transverse axis of the transverse process and a line bisecting the facet joint (Fig. 147.3A).
The pedicle screw is oriented parallel to both the sagittal plane and
the superior and inferior vertebral end plates. Another technique is
the inward method described by Magerl (72,73),
in which the pedicle screw entry point is slightly more lateral than
that described by Roy-Camille. The entrance point is at the junction of
a line intersecting the transverse axis of the transverse process and a
line along the lateral aspect of the facet joint (Fig. 147.3B).
The screw orientation is parallel to the superior and inferior
vertebral end plates but is oriented medially so that it is oblique to
the sagittal plane.
 |
|
Figure 147.3. Three techniques of pedicle screw insertion. A:
The straight-ahead method of Roy-Camille. With this technique, the screw is inserted at the junction of a line bisecting the transverse axis of the transverse process and a line bisecting the facet joint. The pedicle screw is oriented parallel to both the sagittal plane and the superior and inferior vertebral endplates. B: The inward method of Magerl. The pedicle screw entry point is slightly more laterally located than that described by Roy-Camille. The screw entry point is at the junction of a line intersecting the transverse axis of the transverse process and a line along the lateral aspect of the facet joint. The screw orientation is parallel to the superior and inferior vertebral end plates but is oriented medially so that it is oblique to the sagittal plane. C: The author’s preferred up-and-in method, as described by Krag. The entry point for the pedicle screw is at the junction of a line running slightly inferior to the transverse axis of the transverse process and a line along the lateral aspect of the facet joint. The screw is oriented slightly cephalad and is angled medially to the sagittal plane. By making the pedicle screw entry point slightly more caudal than with the other methods, this technique minimizes damage to the superior facet joint by the head of the screw and reduces the risk of subsequent adjacent level degeneration. The screw must be angled superiorly in order to maintain its path within the pedicle. |
The entry point for the pedicle screw is at the junction of a line
running slightly inferior to the transverse axis of the transverse
process and a line along the lateral aspect of the facet joint. The
screw is oriented slightly cephalad and is angled medially to the
sagittal plane. The up-and-in method is particularly useful in
minimizing damage to the superior facet joint. By making the pedicle
screw entry point slightly more caudal than the other methods, damage
to the facet joint by the head of the screw is minimized and the risk
of subsequent adjacent level degeneration is theoretically less. The
screw must be angled superiorly in order to maintain its path within
the pedicle.
pedicle fixation in active, healthy, physiologically young patients
with spinal stenosis associated with degenerative spondylolisthesis who
have relatively few degenerative changes promoting stability at the
level of the slip. I usually manage the elderly, low-demand patient
with multiple comorbidities who has significant associated degenerative
changes at the listhetic level by limited decompression without fusion.
and related only to spinal stenosis decompression. Those that are
specific to only spinal stenosis decompression include complications
related to posterior approaches for spinal stenosis decompression and
those complications associated with spinal fusion.
generally, share certain broad groups of potential complications, which
can be thought of as occurring either preoperatively, intraoperatively, or postoperatively.
surgical outcomes involve primarily surgical decision making, and
therefore, complications of this process can be thought of as being
judgment errors of patient selection. In general, surgery is more
reliable in producing relief of leg pain than LBP. The difficulty with
surgery for LBP lies not with the technical aspects of the surgical
procedures but with the difficulty in determining the genesis of the
back pain. Discography has been advocated as a diagnostic test for
determining the source of pain (22). The role
of discography is controversial and may not accurately predict the
painful level, even when the pain might be coming from the disc (46). See Chapter 144 and Chapter 145 for more details.
comparable to the complications associated with other nonspinal
surgery. These complications include airway complications; fluid
management problems, including shock, fluid overload, and transfusion
reactions; pulmonary complications; cardiac risks related to
perioperative myocardial infarction, cardiogenic shock, or congestive
heart failure; and vascular complications related to blood loss,
hypertension, hypotension, and thrombotic or embolic phenomena.
particular problems related to positioning of the patient in the prone
position, which is the least physiologic position for the patient under
general anesthesia (118). These problems
include potential difficulties with ventilation and airway management.
In addition, there is the risk of pressure to sensitive structures such
as the eyes, which can result in blindness. Pressure can result in
compression of various neural structures, which can result in temporary
or permanent nerve palsies. These structures include the sciatic nerve
or its branches from prolonged pressure of the buttocks against a
buttress while in the kneeling position, the ulnar nerve at the elbow,
the anterior interosseous nerve in the cubital tunnel, the axillary
nerve (84), brachial plexus from excessive shoulder abduction (23), and cervical area from prolonged positioning of the neck in a rotated position.
the abdomen is hanging free in order to reduce inferior vena cava (IVC)
pressure and thereby minimize intraoperative bleeding. This may be
accomplished by placing the patient in a kneeling or a knee-chest
position, or by placing the patient prone with the abdomen hanging
freely. In vivo IVC pressure measurements
have shown that pressure in the IVC is 1.5 times greater when the
patient is in the prone position than when the
patient
is on a frame that allows the abdomen to hang freely. Problems
associated with the kneeling position include sciatic nerve palsy, deep
venous thrombosis (DVT), and compartment syndrome. Ophthalmic
complications associated with spinal surgery have only recently been
recognized and reported (66,82). Such complications include posterior optic nerve ischemia (66,82), occipital lobe infarcts, central retinal vein thrombosis (82), and cerebral ischemia (82).
Although the etiology of the diminished visual acuity or blindness
associated with these conditions is not always clear, identifiable
causes include prolonged operative time, hypotension, blood loss, and
direct pressure on the eye.
persistent spinal fluid leak from spinal needle puncture and
hypotension from venous pooling of blood in the lower extremities. The
advantage of spinal anesthesia in posterior spinal surgery is that some
of the positioning complications previously described can be obviated
by having the patient remain awake and in control of the head and upper
extremities. This approach minimizes the risk of pressure on the eyes,
compression to the ulnar nerve at the elbow, and brachial plexus
palsies.
(SIADH) secretion is a condition characterized by the release of
antidiuretic hormone (ADH) from the posterior pituitary gland in the
absence of the usual osmometric or volumetric stimulus of dehydration
or hypovolemia. This results in failure to excrete free water,
resulting in dilutional hyponatremia. SIADH is known to occur in many
conditions, including surgery, and has also been reported during and
following spinal surgery (7). It is thought
that ADH secretion reaches its maximum during surgery, and that the
syndrome gradually resolves by approximately the third postoperative
day. It is imperative to distinguish between SIADH and hypovolemia as a
cause of low urine output because SIADH demands treatment by fluid
restriction whereas low urine output from hypovolemia requires fluid
administration. SIADH should always be considered as a cause of low
urine output and dilutional hyponaetremia during and immediately after
surgery.
as cauda equina syndrome may predispose the patient to urinary
retention owing to impairment in function of the nervous supply to the
bladder. When urinary retention is due to acute cauda equina
compression, prompt surgery is imperative. Chronic urinary retention
may require either intermittent straight catheterization or an
indwelling catheter. The presence of a urinary catheter, particularly a
long-standing indwelling catheter, may predispose the patient to a
urinary tract infection requiring treatment with antibiotics.
following any surgery, and is common following large posterior spinal
procedures such as multilevel decompressions for spinal stenosis and
instrumented lumbar fusions. When the intertransverse membrane is
violated during posterolateral fusion, bleeding into the
retroperitoneal space may occur, and ileus is more likely.
States, accounting for up to 200,000 deaths annually. In hospitalized
patients, PE is the most common preventable cause of hospital death
with pulmonary emboli detectable in more than one quarter of all
routine autopsies. The etiology of pulmonary embolism includes the
immobilization associated with hospitalization as well as factors
related to surgery itself, which produce a hypercoagulable state. DVT
is the precursor to PE in 90% of cases and is common in hospitalized
patients. The risk of DVT following general surgery ranges between 5%
and 63% and is particularly high with certain orthopaedic conditions,
such as fracture of the hip, and following some orthopedic procedures,
particularly total hip and total knee arthroplasty, in which the
incidence of DVT following unprotected joint replacement is as high as
60% to 80%.
following scoliosis surgery. DVT following routine spinal
decompressions, however, was thought to be a rare occurrence. More
recently, DVT has been recognized following spinal surgery (108,119).
Using postoperative duplex scanning, the incidence of DVT in
unprotected patients undergoing posterior lumbar surgery has been
reported to be 14% (119). The use of elastic
compression stockings or intermittent pneumatic compression stockings
(PCS) has been shown to reduce the incidence of DVT diagnosed by duplex
scanning to 0.9% to 6% (108). Bell et al.
reported the incidence of venographically proven DVT following
unprotected surgery for lumbar disc herniation or spinal stenosis
performed under spinal anesthesia to be 25.8% (6A).
This rate is significantly higher than that reported using duplex
scanning as the method of diagnosis, thereby reflecting the greater
accuracy of venography in diagnosing DVT (30a,30b,119).
Prophylaxis with PCS reduced the incidence to 4.5% in patients
receiving spinal anesthesia. PCS seemed to provide no significant
protection from DVT in patients receiving general anesthesia,
in
whom the incidence of DVT was 13.6% in unprotected spinal surgery and
8.1% with PCS protection. This study suggested that the best
combination of type of anesthesia and DVT prophylaxis in terms of
prevention of DVT was spinal anesthesia with PCS. The worst combination
was spinal anesthesia without PCS. See Chapter 5 for more details as well as recommendations for treatment
soft-tissue, and neural anatomy, and therefore, share a common list of
potential complications. These complications include inadequate neural
decompression, recurrent stenosis, incidental durotomy, neural injury,
epidural hematoma, neural compression from either fat grafts or other
barriers to scar formation, vascular injury, and late instability.
the term, failure to obtain symptomatic relief of radicular leg pain
that is not due to an error in surgical decision making should be
considered at least an adverse effect of surgery. Its avoidance
requires precise correlation of the preoperative imaging study with the
clinical picture and surgical anatomy, and demands that surgery be
continued until the offending neural compression is found. It also
requires a thorough knowledge of surgical anatomy and of the potential
sources and sites of neural compression, as described by MacNab (70).
In addition, it is imperative that the surgeon have a precise
understanding of the potential anatomic variations in the location of
disc herniations so that he will know precisely where to look for
neural compression, particularly when the predicted pathology is not
found (110).
for additional sites of neural compression that may account for
inadequate relief following decompression of only one site. This
condition is sometimes referred to as a “double crush phenomenon” and
is thought to be at least partially due to venous congestion of the
neural segment located between the two sites of compression resulting
in a compartment syndrome–like condition of the intervening segment.
Multiple sites of compression are common with spinal stenosis, which is
a global condition frequently involving multilevel, bilateral neural
compression. Sites of compression include central compression of the
cauda equina and lateral compression, either within the lateral recess,
within the neural foramen, or extraforaminally. It is important to
identify all clinically significant sites of neural compression and to
decompress those levels adequately.
compression from those due to scar formation is a complex
decision-making process that requires a precise history and
high-quality radiographic imaging. Failure to obtain even temporary
pain relief following decompressive lumbar surgery suggests either
inadequate neural decompression, irreversible neural damage already
present at the time of surgery, or a nonspinal cause for the pain. A
short pain-free interval of less than 6 months suggests development of
scar formation as the cause of recurrent pain. Recurrence of pain
following a long pain-free interval of more than 6 to 12 months
suggests a new process such as a recurrent disc herniation or recurrent
stenosis.
disc herniation, recurrence of symptoms following decompression
involving discectomy could be due to recurrent disc herniation. The
overall reported incidence of recurrent disc herniation is
approximately 3% (38). Its incidence following
laminectomy or laminotomy associated with discectomy is unknown but
could be even greater if decompression involved destabilization from
facetectomy and resulted in instability (31).
Although this may be due to many factors, such as associated
comorbidity and advanced patient age, surgical and pathologic factors
are also important. These factors include progression of degenerative
changes at unoperated levels (60), regrowth of bone at the operated levels (90,91), pre-existing instability (degenerative scoliosis or spondylolisthesis) (35),
and development of postoperative instability [for example, due to
resection of one or more facets at a single segmental level (1) or development of a facet fracture at the level of decompression during or following decompression (95)].
All of the above-mentioned factors can lead to recurrent LBP or
radicular leg pain following decompressive surgery for spinal stenosis.
well-recognized complication of spinal surgery that has a reported
incidence of 0.3% to 13%. It most commonly occurs when the edge of a
biting instrument, such as a Kerrison rongeur, inadvertently grabs the
dura and produces either a punctate hole in the dura or a frank
laceration. Usually, the injury is noted immediately by the sudden
appearance of cerebrospinal fluid (CSF) within the wound. Occasionally,
however, the tear is not noted until
sometime
later by the clinical appearance of persistent spinal headache, the
presence of CSF drainage from the wound, or by the onset of an obvious
swelling in the patient’s back suggesting a pseudomeningocele (75). The incidence of the latter complication has been estimated between 0.07 and 2 per cent.
-
It is imperative that there be adequate
exposure to visualize the full extent of the laceration and that
adequate illumination and magnification be available with either an
operating microscope or headlight and loupe magnification. This often
requires wide decompression. -
To facilitate closure of the defect,
place the patient in a slightly head-down (Trendelenberg) position in
order to minimize the amount of CSF in the operative field. This
approach not only provides a drier operative field but also minimizes
the tendency for the individual roots of the cauda equina to float to
the surface, which can result in their inadvertent injury during dural
repair. -
For safe closures of large tears, place a
small cottonoid patty over the exposed nerve roots for the initial
portion of the repair and then remove it just before dural closure (28).
For tears associated with loss of tissue, or for tears in difficult to
repair locations, an autologous fat graft, a piece of autograft fascia
(thoracolumbar fascia or fascia lata), or a freeze-dried fascia
allograft may be required to close the defect (28). Perform a watertight closure with a running 5-0 or 6-0 nonabsorbable suture (e.g., silk or nylon). -
After meticulous closure, return the
patient to a neutral or slightly head-up (reverse Trendelenberg)
position and perform a Valsalva maneuver in order to assess the
integrity of the closure. The use of a fibrin glue may also be
considered for additional strength and integrity of the repair. -
The remainder of the surgical wound
closure proceeds as usual, except that a drain is often not employed in
order to minimize risk of development of a CSF fistula. Oversew the
fascial closure with a running stitch to maintain a watertight closure
of the fascia. Perform routine interrupted skin closure. -
If the dural closure is watertight, the
patient may be ambulatory the day following surgery. If there is any
doubt about the integrity of the closure, keep the patient on bed rest
for 3 to 5 days (28).
persistent spinal headache or a pseudomeningocele, confirm the
diagnosis by myelography or MRI, and return the patient to the
operating room for dural repair and watertight closure (75).
Alternatively, a subarachnoid drain can be inserted at the bedside and
the patient placed on bed rest until the leak subsides. This generally
involves removal of approximately 300 ml of CSF in a sterile blood
collection bag daily. The volume and rate of CSF removal is titrated by
adjusting the height of the bag to produce the appropriate rate of CSF
flow (63).
When identified intraoperatively and repaired primarily, perioperative
surgical morbidity and long-term outcome is comparable to that of
surgery not associated with incidental durotomy (117).
nerve root itself during surgery. This injury may be due to excessive
neural retraction, contusion, laceration, or electrocauterization (111).
The incidence of neurologic complications following lumbar spine
surgery has been estimated to be 0.2%. Such injury may be suspected
postoperatively by the presence of a new or increased objective
neurologic deficit, or by the onset of new parasthesias. This
condition, sometimes referred to as the “battered root,” may occur
either as an unavoidable consequence of severe neural compression or as
a result of indelicate surgery (11). Meticulous
surgical technique, therefore, is of paramount importance in order to
minimize such complications. Adequate surgical exposure is imperative
in order to minimize excessive neural retraction. In cases of a large
midline disc herniation or a disc herniation associated with spinal
stenosis, for example, a bilateral laminectomy rather than a keyhole
laminotomy may be required in order to remove the disc fragment safely.
study of the lumbar spine in order to identify bony anatomy that might
be of surgical significance. Note the presence of spina bifida occulta
or a pre-existing laminectomy defect, for example, on the preoperative
radiograph because the presence of either of these features mandates
cautious surgical exposure in order to minimize risk of damage to
underlying dura and nerve roots. Carefully examine other pre-operative
studies to identify other potentially significant anatomic variants,
such as anomalous nerve roots, that could be injured during surgical
decompression (111).
the nerve root during surgical decompression to be sure that exposure
is not inadvertently being performed in the axilla of a nerve root
where accidental dural laceration and neural injury could occur and
where repair of such injuries is particularly difficult. This is also
important during revision lumbar surgery, in which dissection should
usually be performed lateral to the root along the lateral edge of the
bony canal to avoid a potentially dangerous midline scar. When
performing lateral nerve root decompressions,
work parallel, rather than perpendicular, to the long axis of the nerve root in order to minimize risk of cutting across a root.
surgeries, it has been implicated as a potential cause for continued
pain following spinal surgery. Postoperative scar tissue may be located
either intradurally (arachnoiditis) or extradurally (epidural fibrosis). Arachnoiditis is an inflammation of the pia-arachnoid membrane that surrounds the cauda equina or spinal cord (17).
It can result in surgical failure and continued pain following
decompressive surgery. Its etiology is often unclear, but it has been
associated with many conditions, including oil-based myelographic
contrast agents and prior surgery (93). The
exact mechanism by which arachnoiditis occurs following surgery is not
completely understood, but it is thought to be more likely to occur
following dural laceration in which blood gains entry into the dural
sac and mixes with neural elements. It is also associated with
intraoperative trauma to neural structures. Arachnoiditis exists as a
spectrum of severity, from mild pia-arachnoid thickening to severe
scarring with complete blockage of the flow of contrast agents or
spinal fluid. Diagnosis can be made by water-soluble myelography, MRI,
or post-contrast CT in which the individual nerve roots of the cauda
equina appear clumped together rather than as well-defined structures.
Surgical treatment of arachnoiditis is not indicated because surgery
rarely produces any significant pain relief and may be complicated by
further damage to neural structures and more scarring (17).
scar tissue in which adhesive constrictions can form around neural
tissue. It commonly arises from contact with the paraspinal musculature
and is probably a relatively frequent event following spinal surgery.
Although such scar tissue can result in postoperative pain, symptoms
are relatively infrequent. When postoperative pain exists, the primary
differential diagnosis is between scar and recurrent disc herniation.
Radiographic distinction between these two conditions is best made with
gadolinium-enhanced MRI or post-contrast CT (97).
delicate surgical technique with adequate illumination and
magnification, meticulous hemostasis and drainage, and the use of some
form of an interposition membrane as a barrier to scar formation. These
barriers include a thin layer of fat or synthetic agents such as an
absorbable gelatin sponge. The use of a free fat graft has been
considered the gold standard interposition membrane, although use of
large grafts has been associated with postoperative cauda equina
syndrome (79).
infections. Although the treatment for both types of infections is
often similar (i.e., debridement and antibiotic therapy), it is useful
to make this distinction because duration of treatment (e.g.,
short-term antibiotics for superficial infections versus long-term
intravenous antibiotics for deep infections), morbidity, and long-term
outcome are often very different for the two types.
dermis and subcutaneous tissue but superficial to the deep
thoracolumbar fascia and are characterized by tenderness and localized
erythema. They usually have associated drainage and fluctuance,
although in milder cases consisting only of cellulitis these may be
absent. Patients may be febrile but usually show no other systemic
signs of illness (67). Laboratory data usually
show elevation of the erythrocyte sedimentation rate (ESR) and
C-reactive protein (CRP), although the white blood cell count (WBC) is
usually within normal limits.
local wound care, ranging from simple packing of a small area of
localized infection, followed by a short course of oral antibiotics, to
more aggressive surgical debridement of necrotic tissue with short-term
parenteral antibiotics. In cases requiring surgery, the wound can
usually be closed primarily, although delayed closure is an option if
there is any question about the adequacy of the debridement. The use of
short-term suction-irrigation tubes is at the discretion of the
surgeon, although they are usually not required.
diagnosis is usually readily apparent, deep infections may be difficult
to diagnose and a high index of suspicion is often required (67).
Because delay in diagnosis is common, the amount of tissue necrosis is
often extensive. Symptoms include disproportionate back pain or leg
pain. This may follow a relatively painless and uneventful immediate
postoperative period. The patient may feel and look ill and may exhibit
generalized malaise. Fever is often present but may be deceptively low
grade. If an epidural abscess is present, radicular leg pain and
neurologic deficit may occur. Although the patient may exhibit a
leukocytosis, elevation of the WBC count is frequently absent. The ESR
and CRP are usually elevated.
confirm the diagnosis. MRI provides the best and most useful
information by revealing both the presence and extent of a deep
abscess. Typically, an abscess is demonstrated by the presence of a
well-demarcated area of increased signal intensity on the T2-weighted
image. When
MRI
is not available, diagnosis may be confirmed radiographically by the
presence of a circumscribed area of fluid density visualized by CT. If
a deep abscess is strongly suspected, diagnosis may be confirmed by
aspiration, with subsequent culture and sensitivity of any fluid
obtained (61).
debridement of all necrotic tissues, followed by appropriate parenteral
antibiotics. Begin surgical exposure of the affected area with careful
sequential debridement, and irrigate each layer before proceeding to
the next deeper layer to avoid inadvertent contamination of potentially
unaffected deeper tissues. If the infection extends deeply into the
laminectomy site, take care to remove any fat graft or absorbable
gelatin sponge material. Following removal of infected or suspicious
tissues, thoroughly irrigate the wound with pulsatile lavage. Do not
remove rigid fixation and bone graft from an instrumented spinal fusion
because this may increase the risk of subsequent pseudarthrosis. Loose
hardware, on the other hand, no longer performs its function of
providing stability to the spine and, therefore, should be removed and
thoroughly debrided. Place a drain and close the wound meticulously.
Tightly close the deep fascia with interrupted absorbable suture
oversewn with a continuous running stitch. Close the wound primarily,
particularly in the presence of spinal fixation hardware, unless there
is infection from a particularly virulent organism. This may require
extensive undermining of wound margins in order to avoid tension on
friable wound edges. It is usually advisable to close the wound using
large throws of a sturdy, nonabsorbable suture rather than staples. Use
of suction-irrigation tubes for a few days may be considered, although
this is usually not necessary.
frank paralysis, is one of the most feared complications of spinal
surgery. Fortunately, it is a rare occurrence following spinal surgery,
with only 16% of epidural abscesses resulting from postoperative
infection (4). Signs and symptoms are obvious and constitute a typical presentation (4).
Patients nearly always have significant back pain, and often present
with obvious neurologic findings such as nuchal rigidity and weakness
or paralysis of the lower extremities. The patient appears to be much
sicker than with either postoperative discitis or vertebral
osteomyelitis and typically has a fever. Both the WBC and acute phase
reactants are elevated. MRI is the diagnostic imaging modality of
choice and clearly visualizes the abscess as a discreet,
well-circumscribed entity within the subarachnoid space. It clearly
delineates the upper and lower extent of the abscess and, therefore, is
invaluable in the preoperative planning of the extent of decompression.
decisive: surgical evacuation of the abscess and any adjacent necrotic
tissue, followed by parenteral antibiotics. The preferred surgical
approach is generally posterior, although an anterior approach may be
indicated in the presence of a significant kyphotic deformity in which
bony collapse has compromised the neural structures and simultaneous
anterior reconstruction and bone grafting are required to restore
stability.
compression is another devastating complication of spinal surgery.
Fortunately, the risk of this complication can be minimized by
meticulous attention to preoperative, intraoperative, and postoperative
detail. Preoperatively, advise the patient
to stop all nonsteroidal anti-inflammatory drugs (NSAIDS) for
approximately 1 week before surgery. In addition, it is important that
the patient is not hypercoagulable. When indicated, check the
prothrombin time (PT), partial thromboplastin time (PTT), bleeding
time, platelet count, and platelet function. Intraoperatively,
position the patient with the abdomen hanging freely in order to
minimize epidural venous congestion. Keep the blood pressure below 100
mm Hg systolic, if possible, in order to minimize bleeding. Use
electrocautery, and seal raw bone surfaces with bone wax to minimize
bleeding during the surgical exposure. Control epidural bleeding with
bipolar electrocoagulation. At the end of the surgery, when the deep
paraspinal muscle retractors are removed, check the muscle walls for
persistent bleeding, because prolonged muscle retraction may
temporarily occlude potentially significant muscle bleeders that could
begin bleeding after muscle layer closure. In general, I prefer to use
a drain postoperatively in order to minimize the formation of
postoperative hematoma. Postoperatively,
leave the drain in place for 24 to 48 hours or until the amount of
collected blood is less than approximately 30 ml per 8-hour shift. I do
not prescribe NSAIDS during the immediate postoperative period (during
the first 48 hours) in order to minimize bleeding from the fresh wound.
is the presence of severe pain that appears out of proportion to what
is normally expected. This is usually associated with a progressive
neurologic deficit. Depending on the extent and location of the
surgical exposure and the magnitude of the hematoma, the neurologic
deficit may be focal and unilateral, or it may be widespread and may
involve multiple muscle groups in both legs.
index of suspicion. Confirm the diagnosis with MRI, myelography, or CT.
Once the diagnosis is suspected, immediately return the patient to the
operating room for decompression and drainage of the hematoma.
structures other than epidural hematoma. The use of free fat grafts as
a barrier to scar formation has been associated with symptomatic
neurologic compression mimicking epidural hematoma (79).
Although this risk can be minimized with the use of a smaller (3 to 5
mm thick) piece of fat, the fear of epidural compression by fat graft
has led some surgeons to abandon fat grafts in favor of other synthetic
scar barrier substances. Even these substitutes, however, may cause
neural compression if proper care is not exercised. For multilevel
laminectomy requiring the use of a lengthy piece of scar barrier
material, I prefer an absorbable gelatin sponge material rather than
fat because the fat could theoretically become balled up and exert
focal compression on the underlying dura, and result cauda equina
syndrome.
spinal procedures are nearly always associated with surgical
discectomy, rather than laminectomy. Vascular injury occurs most
commonly at L4–L5, followed by L5–S1 (24).
Although to some extent this reflects the most common levels of spinal
surgery, regional differences in vascular anatomy of the lower lumbar
spine also play a role. Injury of a major abdominal vessel typically
occurs from aggressive use of the pituitary rongeur, with penetration
through the anterior annulus.
bleeding from acute laceration of a major abdominal vessel presents
early as hypotension and abdominal distention and is associated with a
high mortality rate. The mortality rate from arterial injuries has been
reported to be 78%, whereas that for venous injuries is 89% (24).
Vascular injuries may be recognized late by the development of
high-output cardiac failure or abdominal bruits from formation of an
arteriovenous fistula. Arteriovenous fistula formation is the most
common result of a vascular injury. It occurs most commonly between the
right common iliac artery and vein (29.1%), between the left common
iliac artery and vein (25.5%), the right common iliac artery and the
IVC (21.8%), and the right common iliac artery and left common iliac
vein (12.7%). Late arteriovenous fistula formation is more compatible
with long-term survival, with mortality reported between 9% and 11% (24).
an iatrogenic complication of spinal surgery. Such instability can
occur in either the anteroposterior plane (spondylolisthesis), in the
mediolateral plane (lateral listhesis and scoliosis), or in both planes
simultaneously. In general, the risk of postoperative anteroposterior
instability can be minimized by maintaining the integrity of at least
one facet joint at the level decompressed (1).
In other words, if a unilateral complete facetectomy is performed on
one side, then the integrity of the opposite facet must be maintained.
Similarly, if half of one facet is removed during a surgical
decompression, then at least half of the contralateral facet joint
should be spared. If a total of more than one facet is removed during a
decompression, consider prophylactic fusion of that level.
degenerative spondylolisthesis, concomitant fusion should generally be
performed because surgical outcome has been shown to be better with
fusion than with decompression alone (44). This
seems to occur even in the absence of solid bony arthrodesis,
suggesting that even the presence of a stable pseudarthrosis results in
better clinical outcome than when no fusion is attempted. This is
thought to be due to a reduced risk of a subsequent increase in the
slip, although a direct relationship between increase in magnitude of a
subsequent slip and poorer surgical outcome has not been demonstrated.
Although the use of segmental spinal fixation with pedicle screws has
been shown to increase the rate of fusion compared with posterolateral
fusion without instrumentation, there is no convincing evidence that
such instrumentation leads to better clinical outcome (32).
pars interarticularis may also occur in the absence of prior slip as a
result of surgical decompression. In such cases, the patient presents
with evidence of a de novo
spondylolisthesis occurring either at one of the levels decompressed or
at a level above the level of decompression. The presumed mechanism is
either a mechanical stress fracture or perhaps an impairment of the
blood supply to the affected level (16,106). Instability may also occur as a result of fracture of the facet joint following decompressive surgery.
that is largely degenerative in nature, although it may have a
predisposing congenital component to it. It may be accompanied by other
structural changes (degenerative spondylolisthesis) producing
instability. The natural history of spinal stenosis is unclear, but it
appears that approximately 20% of patients worsen over time, 40%
improve slightly, and 40% remain unchanged.
of the symptomatic level or levels. At the time of decompression of
symptomatic levels, the role of surgical decompression of clinically
asymptomatic levels that show compression on imaging studies is
controversial. However, because long-term failures are frequently
characterized by restenosis at previously decompressed levels, or by
the development of symptoms at levels that were previously stenotic but
asymptomatic, it is better to decompress any questionable levels. Such
decompression may be performed through laminectomy or may be more
limited in extent by multilevel laminotomies.
associated spondylolisthesis. In general, fusion is indicated for
spinal stenosis associated with degenerative spondylolisthesis. The
indications for the use of concomitant segmental (pedicle) fixation are
unclear, but we use fixation more commonly in relatively young, active,
healthy patients who lack significant degenerative changes at the level
of the slip. Patients with more severe degenerative changes,
particularly if they are elderly and of low demand, may do well with
focal decompression (laminotomies) without fusion.
scheme: *, classic article; #, review article; !, basic research
article; and +, clinical results/outcome study.
C, Brodsky A, Cauchoix J, et al. Lumbar Spinal Stenosis and Nerve Root
Entrapment Syndromes. Definition and Classification. Clin Orthop 1976;115:4.
GR. Degenerative Lumbar Spinal Stenosis: Natural History and Results of
Simple Decompression and Decompression and Fusion for Degenerative
Spondylolisthesis. In Low Back Pain: A Scientific and Clinical Overview. 1996,663.
GR, Boumphrey F, Piedmont M, et al. The Incidence and Prophylaxis of
Deep Venous Thrombosis (DVT) Following Spinal Surgery. Presented at
20th Annual Meeting of the International Society for the Study of the
Lumbar Spine. Marseilles, France 1993.
J, Logue V. Intermittent Claudication of the Cauda Equina. An Unusual
Syndrome Resulting from Central Protrusion of a Lumbar Intervertebral
Disc. Lancet 1961;1:1082.
SD, Davis DO, Dina TS, et al. Abnormal Magnetic-resonance Scans of the
Lumbar Spine in Asymptomatic Subjects. A Prospective Investigation. J Bone Joint Surg 1990;72A:403.
K, Bridwell KH, Sedgewick TA, O’Brien MF, et al. Role of Fusion and
Instrumentation in the Treatment of Degenerative Spondylolisthesis. J Spinal Disord 1993;6:461.
Q, Baba H, Kamitani K, et al. Postoperative Bone Re-growth in Lumbar
Spinal Stenosis. A Multivariate Analysis of 48 Patients. Spine 1994;19:2144.
DE, Jenkins RS, Bready L, et al. The Prevention of Injuries of the
Brachial Plexus Secondary to Malposition of the Patient During Surgery.
Clin Orthop 1988;228:31.
R, Ciol M, Cherkin D, et al. Lumbar Spinal Fusion. A Cohort Study of
Complications, Reoperations, and Resource Use in the Medicare
Population. Spine 1993;18:1463.
N, Epstein J. Decompression in the Surgical Management of Degenerative
Spondylolisthesis: Advantages of a Conservative Approach in 290
Patients. J Spinal Disord 1998;11:116.
J, Mackay M, Herkowitz H, et al. Degenerative Lumbar Spondylolisthesis
with Spinal Stenosis: A Prospective, Randomized Study Comparing
Decompressive Laminectomy and Arthrodesis with and without Spinal
Instrumentation. Spine 1997;22:2807.
JC, Yaszemski MJ, Lauerman WC, et al. A Randomized Prospective Study of
Posterolateral Lumbar Fusion. Outcomes with and without pedicle screw
instrumentation. Spine 1999;24:553.
D, Lipson S, Fossel A, et al. Associations between Spinal Deformity and
Outcomes after Decompression for Spinal Stenosis. Spine 1997;22:2025.
MA, Howard TC, Gruel CR, et al. Roentgenographic Evaluation of Lumbar
Spine Flexion-extension in Asymptomatic Individuals. Spine 1989;14:327.
H, Garfin S, Bell G, et al. The Use of Computerized Tomography in
Evaluating Non-visualized Vertebral Levels Caudal to a Complete Block
on a Lumbar Myelogram. J Bone Joint Surg 1987;69A:218.
H, Kurz L. Degenerative Lumbar Spondylolisthesis with Spinal Stenosis:
A Prospective Study Comparing Decompression with Decompression and
Intertransverse Process Arthrodesis. J Bone Joint Surg 1991;73A:802.
A, An H, Lim T, et al. Anatomic Changes of the Spinal Canal and
Intervertebral Foramen Associated with Flexion-extension Movement. Spine 1996;21:2412.
RP, Becker GJ, Jacobs RR, et al. The Neuroradiographic Diagnosis of
Lumbar Herniated Nucleus Pulposus: I. A Comparison of Computed
Tomography (CT), Myelography, CT-myelography, Discography, and
CT-discography. Spine 1989;14:1356.
RP, Cain JE Jr, Jacobs RR, et al. The Neuroradiographic Diagnosis of
Lumbar Herniated Nucleus Pulposus: II. A Comparison of Computed
Tomography (CT), Myelography, CT-myelography, and Magnetic Resonance
Imaging. Spine 1989;14:1362.
K, Uden A, Rosen I. The Effect of Decompression on the Natural Course
of Spinal Stenosis. A Comparison of Surgically Treated and Untreated
Patients. Spine 1991;16:615.
B, Annertz M, Sjoberg C, et al. A Prospective and Consecutive Study of
Surgically Treated Lumbar Spinal Stenosis. Part I: Clinical Features
Related to Radiographic Findings. Spine 1997;22:2932.
B, Stromqvist B. Symptoms and Signs in Degeneration of the Lumbar
Spine. A Prospective, Consecutive Study of 300 Operated Patients. J Bone Joint Surg 1993;75:381.
J. Comorbidity and Outcome in Degenerative Lumbar Spinal Stenosis. Low
Back Pain: A Scientific and Clinical Overview. 1996;41:689.
J, Dalgas M, Stucki G, et al. Degenerative Lumbar Spinal Stenosis.
Diagnostic Value of the History and Physical Examination. Arthritis Rheum 1995;38:1236.
J, Lipson S, Brick G, et al. Clinical Correlates of Patient
Satisfaction after Laminectomy for Degenerative Lumbar Spinal Stenosis.
Spine 1995;20:1155.
SH, Eismont FJ, Green BA. Closed Subarachnoid Drainage for Management
of Cerebrospinal Fluid Leakage after an Operation on the Spine. J Bone Joint Surg 1989;71A:984.
H, Kanamori M, Ishihara H, et al. Expansive Lumbar Laminoplasty for
Degenerative Spinal Stenosis in Patients below 70 Years of Age. Eur Spine J 1997;6:191.
S, Sakou T, et al. Natural History of Degenerative Spondylolisthesis:
Pathogenesis and Natural Course of the Slippage. Spine 1990;15:1204.
P, Jacobsen F. Cauda Equina Syndrome after Surgical Treatment of Lumbar
Spinal Stenosis with Application of Free Autogenous Fat Graft. A Report
of Two Cases. J Bone Joint Surg 1989;71:1090.
J. Microdecompression and Uninstrumented Single-level Fusion for Spinal
Canal Stenosis with Degenerative Spondylolisthesis. Spine 1998;23:2243.
O, Ookawa A, Yamaura I. Long-term Roentgenographic and Functional
Changes in Patients Who Were Treated with Wide Fenestration for Central
Lumbar Stenosis. J Bone Joint Surg 1991;73A:1184.
SE, Serena SH, Workman KL, et al. Patient Outcomes after Decompression
and Instrumented Posterior Spinal Fusion for Degenerative
Spondylolisthesis. Spine 1999;24:561.
K, Rydevik B, Holm S. Edema Formation in Spinal Nerve Roots Induced by
Experimental, Graded Compression. An Experimental Study on the Pig
Cauda Equina with Special Reference to Differences in Effects between
Rapid and Slow Onset of Compression. Spine 1989;14:569.
F, Cinotti G, Perugia D, et al. The Surgical Treatment of Central
Lumbar Stenosis. Multiple Laminotomy Compared with Total Laminectomy. J Bone Joint Surg 1993;75B:386.
with Roy-Camille Fixation for the Thorocolumbar and Lumbar Spine. Acute
Spinal Injuries: Current Management Techniques. University of
Massachusetts Continuing Medical Education Course. Sturbridge, MA: University of Massachusetts, 1987.
BL, Pedowitz RA, Hargens AR, et al. Effects of Acute, Graded
Compression on Spinal Nerve Root Function and Structure. An
Experimental Study of the Pig Cauda Equina. Spine 1991;16:487.
WO, Spratt KF, Weinstein J, et al. The Consistency and Accuracy of
Roentgenograms for Measuring Sagittal Translation in the Lumbar
Vertebral Motion Segment: An Experimental Model. Spine 1990;15:741.
J, Silveri C, Balderston R, et al. The Results of Operations on the
Lumbar Spine in Patients Who Have Diabetes Mellitus. J Bone Joint Surg 1993;75:1823.
M, Bressler E, Lonstein J, et al. Deep Venous Thrombosis and Pulmonary
Embolism after Major Reconstructive Operations on the Spine. A
Prospective Analysis of Three Hundred and Seventeen Patients. J Bone Joint Surg 1994;76:980.
A, Garfin S. Degenerative Lumbar Spondylolisthesis with Spinal
Stenosis, a Prospective Study Comparing Decompression with
Decompression and Intertransverse Process Arthrodesis: A Critical
Analysis. Spine 1997;22:368.
JC, Bohlman HH, Riew KD. Dural Tears Secondary to Operations on the
Lumbar Spine. Management and Results After a Two-Year-Minimum Follow-up
of Eighty-eight Patients. J Bone Joint Surg 1998;80A:1728.
SW, Tsourmas N, Feffer HL, et al. A Study of Computer-assisted
Tomography: I. The Incidence of Positive CAT Scans in an Asymptomatic
Group of Patients. Spine 1984;9:549.
M, Shima K, Taniguchi Y, et al. Hypertrophied Ligamentum Flavum in
Lumbar Spinal Canal Stenosis. Pathogenesis and Morphologic and
Immunohistochemical Observation. Spine 1992;17:1353.
S, Veerapen R, O’Laoire S. Relief of Lumbar Canal Stenosis Using
Multilevel Subarticular Fenestrations as an Alternative to Wide
Laminectomy: Preliminary Report. Neurosurgery 1988;23:628.
HA, Garfin SR, Dickman CA, Mardjetko SM. A Historical Cohort Study of
Pedicle Screw Fixation in Thoracic, Lumbar, and Sacral Spinal Fusions. Spine 1994;19(Suppl):2279S.
